TNF alpha & Inflammation
Delving into TNF alpha's critical role in inflammation and its therapeutic targeting.
Key Takeaways:
- TNF alpha is a crucial cytokine in inflammation and apoptosis.
- Its structure facilitates interaction with TNF receptors, influencing immune responses.
- Elevated TNF alpha levels are linked to various inflammatory diseases.
- TNF alpha activates distinct pathways through TNFR1 and TNFR2, impacting cell function.
- Its involvement in diseases like rheumatoid arthritis and psoriasis highlights its clinical significance.
- Therapeutic targeting of TNF alpha offers potential in treating inflammatory conditions and cancer.
What is TNF alpha?
Tumour necrosis factor alpha (TNF alpha) is a transmembrane protein (molecular weight of TNF alpha - 26 kDa) and a member of the TNF superfamily, with its 17 kDa secreted form mediating intracellular signalling. Originally identified in 1975 as an endotoxin-induced glycoprotein in macrophages, and later determined to be ubiquitously expressed in multiple cell types (Carswell et al., 1975), TNF alpha stimulates several critical inflammatory and apoptotic pathways by binding to one of its two membrane bound receptors, TNFR1 and TNFR2 (Aggarwall, 2003). TNF alpha levels are not detectable under healthy conditions in humans, however elevated levels of TNF alpha in tissues and serum are found in inflammatory conditions (Robak et al, 1998). TNF alpha was first cloned in 1984, and in the following two decades it has become the focus of both in vivo and in vitro experimentation, with a critical focus on its role in inducing tumourigenesis (Burugu et al, 2017; Wang et al, 2016; Pennica et al, 2005).
TNF Alpha Structure
Tumor Necrosis Factor alpha (TNF-alpha) is a homotrimeric cytokine composed of three identical subunits, each with alpha-helices and beta-sheets. Disulfide bonds stabilize the tertiary structure, and it possesses receptor-binding sites to interact with TNF receptors (TNFRs), initiating inflammation, apoptosis, and immune responses. Post-translational modifications like glycosylation can occur, and excessive TNF-alpha activity is associated with inflammatory and autoimmune diseases. Drugs targeting TNF-alpha have been developed to manage these conditions effectively.
3D Structure of TNF Alpha - Source: PDB
Function of TNF Alpha
Tumor Necrosis Factor alpha (TNF-alpha) is a vital cytokine with diverse functions in the immune system. Primarily produced by immune cells, it plays a key role in regulating inflammation and immune responses. When infections, injuries, or other harmful stimuli are detected, TNF-alpha is released, initiating the inflammatory cascade by recruiting immune cells to the affected site. It also aids in the activation of macrophages and neutrophils to combat invading pathogens. Moreover, TNF-alpha can induce apoptosis (programmed cell death) in specific cells, contributing to the elimination of infected or damaged cells. However, dysregulated TNF-alpha production can lead to chronic inflammation and autoimmune diseases. Targeted therapies, such as TNF inhibitors, have been developed to manage inflammatory conditions by controlling TNF-alpha activity effectively.
TNF Alpha Receptors and Signalling Cascades
TNF alpha exhibits similar affinity for both of its receptors, but their distribution and functions differ. TNFR1 is expressed on various cell types, while TNFR2 is primarily found on cells of the haematopoietic lineage, including specific T cell subsets, as well as on immune-signaling endothelial cells and mesenchymal stem cells (Naudé et al., 2007; Aggarwal et al., 2003). The regulation of TNF secretion and processing of TNF receptors is mediated by an enzyme called TNF-converting enzyme, or TACE. This enzyme plays a crucial role in modulating the pro- or anti-inflammatory functions of TNF alpha and its receptors on a cell-specific level (Black et al., 1997).
The cytoplasmic portion of TNFR1 contains a death domain, which initiates downstream signaling through the caspase cascade. In contrast, TNFR2 lacks a death domain and directly binds to TNF-receptor-associated factor 1 (TRAF1) or TRAF2 at the cell membrane (Nagata et al., 1995). Both TNFR1 and TNFR2 contribute to inflammatory pathways by inducing the activation of NF-Kappa Beta and mitogen-associated protein kinase (MAPK) (Ledgerwood et al., 1999).
The distinct characteristics of TNFR1 and TNFR2, including their cell-specific expression and signaling mechanisms, highlight the complexity of TNF alpha's interactions within the immune system. Understanding these differences is crucial for unraveling the precise roles of TNF alpha and its receptors in inflammatory processes and developing targeted therapeutic interventions.
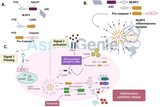
TNF Alpha Signalling Pathway
TNF Alpha and Apoptosis
Ligand binding to TNFR1 induces apoptosis via the caspase cascade. Specifically, the cytoplasmic death domain of TNFR1 recruits TNFR-associated death domain protein (TRADD) which forms a complex with Fas-associated death domain (FADD) and caspase 8, preceding either extrinsic or intrinsic apoptosis (mitochondrial-mediated). Extrinsic apoptosis involves the cleavage and subsequent activation of caspase 3 by active caspase 8. Intrinsic apoptosis is facilitated via the truncated form of the pro-apoptotic Bcl-2 protein Bid, leading to the translocation of Bax and Bak to the mitochondrial membrane which induces Cytochrome-c release and the formation of the apoptosome. Both extrinsic and intrinsic apoptotic pathways culminate in caspase-3 activation (Kantari and Walczak, 2011; Wang et al, 1996). As TNFR2 is death domain free, it does not induce apoptosis alone, however there is some evidence for crosstalk of both TNFR1 and TNFR2 (Naudé et al, 2007).
TNF Alpha and Inflammatory Pathways
Ligation of TNFR1 and -2 also induce inflammatory pathway activation. TRADD association with TRAF2 and Receptor-interacting protein (RIP1) induce downstream IKK-complex activation, IKappa Beta alpha phosphorylation and degradation and the release of NF-Kappa Beta subunits inducing nuclear translocation and NF-Kappa Beta activation. Similarly, TRADD mediated recruitment of TRAF2 can activate MAPK pathways, including c-Jun N-terminal kinase (JNK) and p38, which result in activation of the transcription factor AP-1 (Blonska et al, 2005; Devin et al 2001). Additionally, there is crosstalk between inflammatory and apoptotic pathways, evident in TNF alpha-induced NF-Kappa Beta activation, which can regulate apoptosis by upregulating cFLIP levels, which in turn inhibit caspase 8 activation (Micheau et al, 2001).
TNF Alpha in Inflammatory Disease and Cancer
Importantly, acute inflammation is a therapeutic and preventative natural defense; however, chronic inflammation is detrimental and drives a plethora of pathological conditions. TNF alpha has been identified as the critical mediator of the endotoxin-induced response, specifically facilitating that of Escherichia coli and tnfr1-/-mice are resistant to lipopolysaccharides and Staphylococcus aureus (Pfeffer et al, 1993; Beutler et al, 1985). The inflammatory effects of TNF alpha are primarily mediated via NF-Kappa Beta. Chronic inflammation is now classified as a precursor and leading risk factor to tumorigenesis, with inflammatory signalling in the tumour microenvironment promoting tumour progression and resistance (Balkwill et al, 2005; Karin et al, 2005). Moreover, TNF alpha has been identified to play a crucial role in tumour initiation and development (Aggarwal et al, 2002; Sungamuma et al, 1999; Deigel et al, 1989). One of the mechanisms employed by TNF alpha which promotes tumour development is the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which in turn lead to DNA damage (Woo et al, 2000). Additionally, TNF alpha induces angiogenesis of tumour cells by upregulating several angiogenic factors such as vascular endothelial growth factor (VEGF) (Jing et al, 2011). Furthermore, TNF alpha promotes motility of epithelial tumour cells (Rosen et al, 1991). However, the role of TNF alpha in carcinogenesis is complex due to the range of signalling responses described above, which effectively lead to activation of 4 distinct pathways: a death domain induced pro-apoptotic pathway; an anti-apoptotic pathway, facilitated by TRAF2 associations with the E3 ubiquitin ligase cellular inhibitor of apoptosis-1 (cIAP1); MAPK-mediated AP-1 activation, and RIP1 induced NF-Kappa Beta (Chen and Goeddel, 2002). The current consensus eludes to the TNF alpha tumour microenvironment concentration and cell-specific site of elevated expression (Ohri et al, 2010).
Table 1. Overview of the involvement of TNF alpha in various inflammatory conditions.
| Inflammatory Disease | Role of TNF alpha |
| Rheumatoid Arthritis | TNF alpha is a key driver of joint inflammation and destruction. It promotes synovial inflammation, cartilage degradation, and bone erosion |
| Inflammatory Bowel Disease | TNF alpha plays a crucial role in the pathogenesis of Crohn's disease and ulcerative colitis. It contributes to intestinal inflammation, mucosal damage, and perpetuates the inflammatory cascade |
| Psoriasis
| TNF alpha is involved in the immune dysregulation and abnormal skin cell proliferation seen in psoriasis. It promotes keratinocyte activation, angiogenesis, and recruitment of inflammatory cells to the skin |
| Psoriatic Arthritis | TNF alpha is implicated in the joint inflammation and structural damage seen in psoriatic arthritis. It drives synovial inflammation, cartilage degradation, and bone erosion |
| Noninfectious Uveitis | Elevated levels of TNF alpha are often detected in the aqueous humor and ocular tissues of uveitis patients, contributing to the recruitment and activation of inflammatory cells within the eye. |
Therapeutic potential of targeting TNF Alpha
TNF alpha offers a prime therapeutic target for cancer. To date, several drugs have been developed which target and inhibit TNF alpha, including inhibitors of TNF alpha expression (Majumdar et al, 2002); TNF alpha antibodies which bind and neutralize TNF alpha, for example infliximab, although several of these have been shown to have adverse effects (Keystone et al, 2004); and inhibitors of TNF alpha signalling primarily targeting NF-Kappa Beta (Aggarwal et al, 2006). Several methods of tumour modulation targeting TNF alpha, among other cytokines, include adoptive T cell therapy, where patients’ dendritic cells are stimulated with TNF alpha in order to induce antigen presentation (Yee et al, 2002). A similar principal has been applied for the development of dendritic cell targeted cancer vaccines which have proven to have some success in clinical trials (Ridgway, 2003). Overall, targeting cytokines, particularly TNF alpha which has the opposing roles of apoptosis and cell survival signalling, is a field of huge interest and potential with relation to cancer therapeutics and treatments for inflammatory disorders.
Figure 1: TNFa singalling pathways. TNFa binds to the membrane-bound receptors TNFR1 or TNFR2, leading to downstream apoptosis and inflammatory signalling. Activation of TNFR1 induces the formation of a death inducing signalling complex, containing TRADD, FADD and caspase-8. Formation of this complex leads to the induction of apoptosis by either the intrinsic or extrinsic pathways, both culminating in the cleavage of caspase-3. Pro-inflammatory singalling pathways can also be induced by either TNFR1 or TNFR2 activation, which signal through the adaptor protein TRAF2 to activate RIP1, NIK or MEKs, resulting in the activation of MAPK and NF-kB activation.
TNF Alpha Inhibitors/ TNF Alpha Antagonists
Table 2. Overview of some commonly used TNF alpha inhibitors and their general mechanisms of action
| TNF alpha Inhibitor | Mechanism of Action |
| Infliximab (Remicade®) | Monoclonal antibody that binds to soluble and transmembrane TNF alpha, preventing it from binding to its receptors. It promotes apoptosis of TNF alpha-expressing cells and reduces inflammation. |
| Etanercept (Enbrel®) | Fusion protein consisting of the extracellular domain of TNF receptor 2 (TNFR2) and the Fc portion of human IgG1. It acts as a decoy receptor, binding to TNF alpha and preventing it from interacting with cell surface TNF receptors |
| Adalimumab (Humira®) | Monoclonal antibody that specifically targets TNF alpha. It binds to soluble and transmembrane TNF alpha, inhibiting its interaction with TNF receptors and modulating the inflammatory response |
| Certolizumab Pegol (Cimzia®) | Pegylated Fab fragment of a humanized monoclonal antibody against TNF alpha. It binds to soluble TNF alpha and prevents it from binding to TNF receptors, thereby reducing inflammation |
| Golimumab (Simponi®we) | Fully human monoclonal antibody that selectively targets soluble and transmembrane TNF alpha. It inhibits the binding of TNF alpha to its receptors and reduces inflammation |
References
- Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC.The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr Drug Targets Inflamm Allergy. 2002. 1(4):327-41.
- Aggarwal BB, Shishodia S, Takada Y, Jackson-Bernitsas D, Ahn KS, Sethi G, Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006;(56):161-86.
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003. 3(9):745-56.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005. 7(3):211-7. - Beutler BA, Milsark IW, Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985. 135(6):3972-7.
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997. 385(6618):729-33.
- Blonska M, Shambharkar PB, Kobayashi M, Zhang D, Sakurai H, Su B, Lin X. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem. 2005. 280(52):43056-63.
- Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2017 10.1016/j.semcancer.2017.10.001.
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975. 72(9):3666-70.
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002. 296(5573):1634-5.
- Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Zg. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001. 21(12):3986-94.
- Digel W, Stefanic M, Schöniger W, Buck C, Raghavachar A, Frickhofen N, Heimpel H, Porzsolt F. Tumor necrosis factor induces proliferation of neoplastic B cells from chronic lymphocytic leukemia. Blood. 1989. 73(5):1242-6.
- Jing Y, Ma N, Fan T, Wang C, Bu X, Jiang G, Li R, Gao L, Li D, Wu M, Wei L.Tumor necrosis factor-alpha promotes tumor growth by inducing vascular endothelial growth factor. Cancer Invest. 2011. 7:485-93.
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011. 1813(4):558-63.
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005. 5(10):749-59.
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA, Chartash EK. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004. 50(5):1400-11.
- Ledgerwood EC, Pober JS, Bradley JR. Recent advances in the molecular basis of TNF signal transduction. Lab Invest. 1999. 79(9):1041-50.
- Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol. 2002. 168(6):2644-51.
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001. 21(16):5299-305.
- Nagata S, Golstein P. The Fas death factor. Science. 1995. 267(5203):1449-56.
- Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011. 278(6):888-98.
- Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer. 2010. 10:323.
- Pennica D, Hayflick JS, Bringman TS, Palladino MA, Goeddel DV. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985. 82(18):6060-4.
- Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993. 73(3):457-67.
- Ridgway D. The first 1000 dendritic cell vaccines. Cancer Invest. 2003;21(6):873-86.
- Robak T, Gladalska A, Stepień H. The tumour necrosis factor family of receptors/ligands in the serum of patients with rheumatoid arthritis. Eur Cytokine Netw. 1998. 9(2):145-54.
- Rosen EM, Goldberg ID, Liu D, Setter E, Donovan MA, Bhargava M, Reiss M, Kacinski BM. Tumor necrosis factor stimulates epithelial tumor cell motility. Cancer Res. 1991. 51(19):5315-21.
- Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999. 59(18):4516-8.
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996. 10(22):2859-69.
- Wang P, Wang J, Yu M, Li Z. Tumor Necrosis Factor-α T-857C (rs1799724) Polymorphism and Risk of Cancers: A Meta-Analysis. Dis Markers. 2016; 2016:4580323. doi: 10.1155/2016/4580323. Epub 2016 Dec 27.
- Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, Kim JH. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem. 2000. 275(41):32357-62.
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002. 99(25):16168-73.
Top ELISA Kits
Written by Sinéad Kinsella PhD
Sinéad Kinsella completed her PhD on the innate immune system in Amyotrophic Lateral Sclerosis (ALS). She now works as a Immunotherapy & Immunometabolism Scientist at Fred Hutch.
Additional Resources
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024