Rapid COVID Antibody Test | Antibody Tests
What does the COVID-19 Rapid POC CE-IVD Test detect?
The test detects the presence of patient-generated antibodies against spike protein, the virus which causes the disease COVID-19. The test can detect two types of antibody isotypes: IgG and IgM. IgM antibodies are the first antibodies to appear in response to a novel antigen. They imply a more recently initiated infection. IgG antibodies have a higher affinity for the target antigen, meaning they are more specifically able to bind the substance which caused the immune response. IgG antibodies are generated later in the course of infection. IgM and IgG antibodies can both be present in a sample. This implies that the conversion from a primarily IgM to IgG humoral response is underway. A sample can be positive if there are IgM, IgG, or both IgM and IgG antibodies present.
Key Takeaways
- The COVID-19 Rapid POC CE-IVD Test detects patient-generated antibodies (IgG and IgM) against the SARS-CoV-2 spike protein.
- It employs a lateral flow immunoassay principle, similar to a pregnancy test, to qualitatively assess the presence of these antibodies.
- Sensitivity and specificity are critical factors determining the test's accuracy.
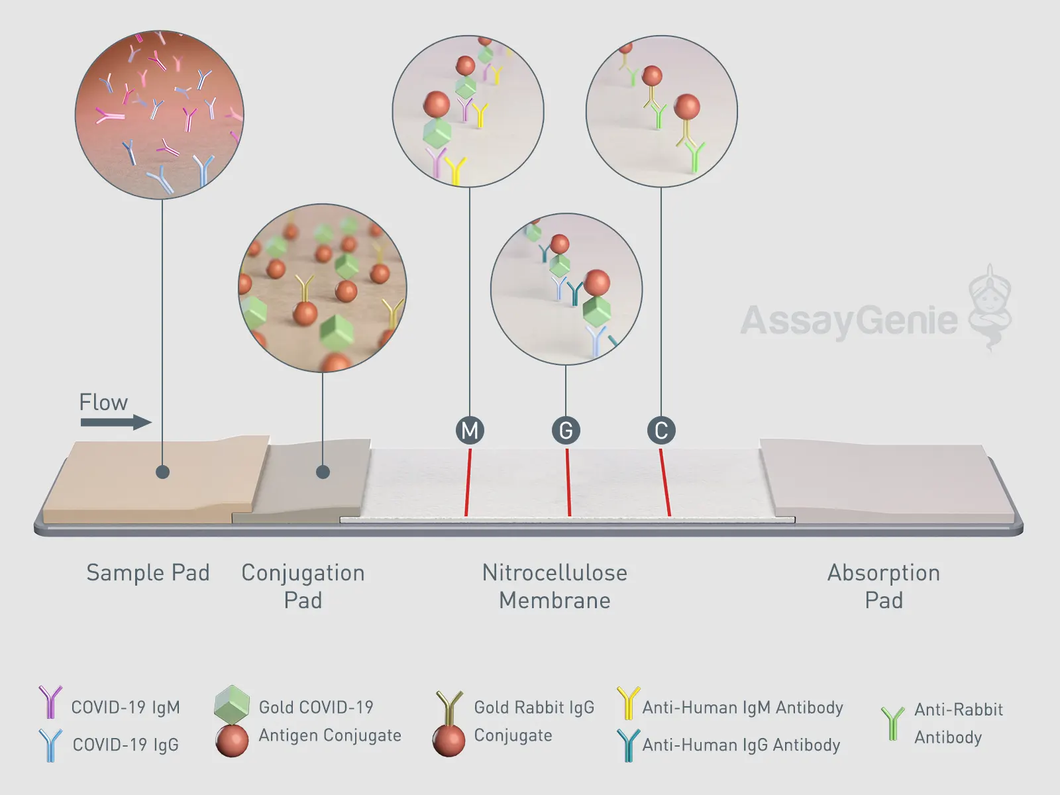
COVID-19 Detection Cassette Details
Fig 1. COVID-19 Detection Test Strip, labelled with major functional areas.
Top ELISA Kits
How does the COVID-19 Rapid POC CE-IVD Test work?
Overview
The COVID-19 Rapid POC CE-IVD test is a lateral flow immunoassay. A lateral flow immunoassay is a way to qualitatively assess the presence of an analyte from a patient sample or specimen. In this case, the analytes being detected are IgG and IgM antibodies specific for SARS-CoV-2.
The IgG/IgM test cassette is a qualitative membrane-based immunoassay for the detection of IgG and IgM antibodies to 2019-nCoV in whole blood, serum or plasma specimens.
The principle of this test is similar to that of a hCG pregnancy test, which also uses a rapid chromatographic immunoassay for qualitative detection of a human glycoprotein.
Components
Included:
- Test cassette: the functional unit of the test which holds the immunoassay strip inside.
- Droppers: used to transfer the samples to the specimen well.
- Package insert: contains the full technical manual with instructions and further specifications.
- PBS buffer solution: added to the specimen well to facilitate the test.
Other:
- Specimen collection containers: used to hold samples after collection.
- A centrifuge (for plasma only) and lancets (for fingerstick whole blood only)
- Capillary tube: necessary if a fingerstick whole blood specimen is to be used.
- Pipettes: can be used in place of droppers.
- Timer: to track the 10 minute wait time.
COVID-19 Rapid POC CE-IVD Test: Lateral Flow Detection Steps
Fig 2. A schematic of the COVID-19 lateral flow test.
Steps:
- The whole blood, serum, or plasma clinical specimen is added to the well. Then, the dilution buffer (10 mM phosphate-buffered saline) is added.
2. The combined sample flows down to the sample pad.
3. Capillary action/lateral flow will move the sample across the test.
4. The sample will hit the conjugation pad. The conjugation pad contains the COVID-19 antigen conjugated to 40 nm gold nanoparticle (AuNP) colloid. During this stage, any antibodies in the sample with specificity for COVID-19 will bind the antigen and its conjugated gold nanoparticle. (The kit control, rabbit IgG, is conjugated to the same gold nanoparticle and travels with the rest of the sample from this step.)
5. Next, the sample/conjugate complex moves to the nitrocellulose membrane. Here, it comes in contact with the three test lines: IgG, IgM and control.
6. First is the M line, which contains an immobilised antibody that recognises human IgM. Any IgM antibodies will bind here. However, only human IgM antibody/COVID-19 antigen/gold nanoparticle complexes will produce a visible coloured line.
7. Second is the G Line, which contains an immobilised antibody that recognises Human IgG. All IgG antibodies will bind here. However, only human IgG antibody/COVID-19 antigen/gold nanoparticle complexes will produce a visible coloured line.
8. The control line is the last line the sample will encounter. The control line contains an immobilised antibody that recognises Rabbit IgG, the control antibody. To serve as a procedural control, a coloured line should always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
9. Finally, any excess will flow through to the absorption pad.
10. After 10 minutes, the results of the test can be read.
COVID-19 Rapid POC CE-IVD Test Principle
This COVID-19 Rapid Point of Contact CE-IVD test consists of two components, an IgG and an IgM. In the IgG component, anti-human IgG coats the G test line region. In the IgM component, anti-human IgM coats the M test line region.
During testing, the specimen reacts with SARS-CoV-2 antigen-coated gold nanoparticles (AuNP) in the conjugation pad of the test cassette. Any antibody in the patient sample that recognises the MK201027 SARS-CoV-2 antigen binds to the Antigen-AuNP complex. The mixture then migrates laterally across the membrane by capillary action/lateral flow.
As these human antibody/antigen/AuNP complexes move across the test lines, they are captured at the anti-human IgM 'M' Line, the anti-human IgG 'G' Line, or both, depending on the antibody contents of the specimen.
The sample first reaches the anti-human IgM antibodies which coat the M line. If the specimen contains IgM antibodies to SARS-CoV-2, a coloured line will appear in the M test line region.
Next, the sample reaches the anti-human IgG antibodies which coat the G line. If a specimen contains IgG antibodies to SARS-CoV-2, the conjugate-specimen complex reacts with anti-human IgG. A coloured line appears in the G test line region as a result.
Only human antibody/SARS-CoV-2 Antigen/AuNP complexes will produce a visible red or pink line at the M or G Line. Other antibodies produce no colour.
The rabbit IgG-AuNP complexes are captured by the control line (which contains anti-rabbit-IgG). This visible line indicates that there has been successful lateral flow across the detection strip. It is last to ensure that the sample had sufficient volume to move across the entirety of the test cassette.
Excess antigen-AuNP complexes will not be captured by the M or G lines. If no anti-MK201027 antibodies are present in a patient sample, no Ag-AuNP complex will be captured at the M or G Lines, and thus no coloured line will appear.
To summarise, if the specimen contains SARS-CoV-2 IgG antibodies, a coloured line will appear in IgG test line region. If the specimen contains SARS-CoV-2 IgM antibodies, a coloured line will appear in IgM test line region. If the specimen does not contain SARS-CoV-2 antibodies, no coloured line will appear in either of the test line regions, indicating a negative result. In all cases, a coloured line should appear at the control, C line.
Is the COVID-19 Rapid POC CE-IVD Test accurate?
Accuracy is derived from two key metrics: sensitivity and specificity.
Sensitivity is defined as the proportion of true positives which are correctly identified by the test (eg. a person has IgG antibodies in their sample, and the test detects those antibodies). It is also sometimes called the probability of detection.
Specificity is defined as the proportion of true negatives that are correctly identified by a test.
The data for IgG and IgM specificity and sensitivity is below.
Fig 3. IgG Relative Sensitivity: 100% (95%CI*: 86.0%-100%). Relative Specificity: 98.0% (95%CI*: 89.4%-99.9%). Accuracy: 98.6% (95%CI*: 92.3%-99.96%) *Confidence Interval
Fig 4. IgM Relative Sensitivity: 85.0% (95%CI*: 62.1%-96.8%). Relative Specificity: 96.0% (95%CI*: 86.3%-99.5%). Accuracy: 92.9% (95%CI*: 84.1%-97.6%)
Control
A control measurement is in place to ensure the test is functioning correctly. It confirms sufficient specimen volume and correct procedural technique. Any test that is missing the control line (‘C’) is invalid and should be performed again. If possible, it is always good laboratory practice to run a positive and negative control to verify proper test performance prior to diagnostic testing.
SARS-CoV-2 antibodies
| SKU | Product Name | Conjugation | Application |
CAB20022 | Unconjugated | ELISA | |
AGEL2111 | Unconjugated | WB,ELISA |
SARS-CoV-2 recombinant proteins
| SKU | Product Name | Conjugation | Region | Species |
CARP01258 | His Tag | Arg319-Phe541 | SARS-CoV-2 | |
CARP01259 | His Tag | Val11-Arg682 | SARS-CoV-2 | |
CARP01260 | His Tag | Val11-Gln1208 | SARS-CoV-2 |
Recent Posts
-
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024 -
Biomarker Testing: Advancements, Applications, and Future Directions
Biomarkers, measurable indicators of biological processes or responses to therapeutic interve …14th Apr 2024