Anti-Infliximab (Remsima®)ADA Quantitative ELISA Kit
- SKU:
- HUMB00007
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Infliximab (Remsima®)
- Research Area:
- Anti-TNF Alpha
Description
Anti-Infliximab ADA Quantitative ELISA Kit
Enzyme-linked immunosorbent assay for the quantitative determination of antibodies to infliximab Remsima® in serum and plasma. Remsima® is the world's first biosimilar mAb (approved in 2013 by EMA). The Agencys Committee for Medicinal Products for Human Use (CHMP) decided that, in accordance with EU requirements, Remsima® has been shown to have a comparable quality, safety and efficacy profile to Remicade. Infliximab (Remsima®) was associated to the development of anti-infliximab antibodies, even some were reported to be neutralizing, in various percentages of patients during therapy with the drug Remsima®. This could lead to severe complications. The Assay Genie Antibody to Infliximab® ELISA Kit can be used to monitor Infliximab anti-drug antibodies (ADA) and is for research use only.
Anti-Infliximab (Remsima®) ADA Quantitative ELISA Kit test principle
The Assay Genie Antibody to infliximab biosimilar (CT-P13) (Remsima®) ELISA is a sandwich assay for the determination of antibodies against infliximab biosimilar (CTP13) in serum and plasma samples. During the first incubation period, antibodies to infliximab biosimilar (CT-P13) in patient serum/plasma samples are captured by the drug infliximab biosimilar (CT-P13) (Remsima®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase-labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-infliximab antibodies in the sample.
Anti-Infliximab (Remsima®) ADA Quantitative ELISA Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 31 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Tumour Necrosis Factor Alpha Remsima |
Anti-Infliximab (Remsima®) ADA Quantitative - Key Information
Infliximab mode of action
Infliximab (Remsima®) is a chimeric monoclonal antibody and used to treat autoimmune disorders. Infliximab reduces the amount of active human tumour necrosis factor alpha (hTNF alpha) in the body by binding to it and preventing it from signalling the receptors for TNF alpha on the surface of various cell types. TNF alpha is one of the key cytokines that triggers and sustains the inflammatory reactions.
Infliximab uses
Remsima, the world’s first biosimilar mAb (approved in 2013 by EMA). The Committee for Medicinal Products for Human Use (CHMP) decided that, in accordance with EU requirements, Remsima presented comparable quality, safety and efficacy profile to Remicade. Infliximab (Remsima®) is a tumor necrosis factor alpha (TNF-alpha) antagonist used to treat rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, adult Crohn's disease, plaque psoriasis, and psoriatic arthritis.
Infliximab immunogenicity
One of the major concerns, despite its wide usage, is the potential development of anti-infliximab antibodies which in turn may interfere with infliximab (Remsima®) efficacy. This was determined based on the return of signs and symptoms of disease. This issue could lead to a dose-escalation of the therapeutic or a potential termination of treatment. In this context, the presence of anti-infliximab antibodies during treatment with infliximab (Remsima®) is a major concern and monitoring for the presence and/or quantitation of specific antibodies during clinical trials is often a necessity for follow up of the treatment regimens.
The Assay Genie anti-infliximab antibody ELISA Kit can be efficiently used for monitoring infliximab-specific antibodies during therapy and is for research use only. With this Assay Genie ELISA test, anti-infliximab antibodies can be detected in biological samples.
Anti-Infliximab ADA Quantitative ELISA Kit Contents
| Size | Kit contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with biosimilar |
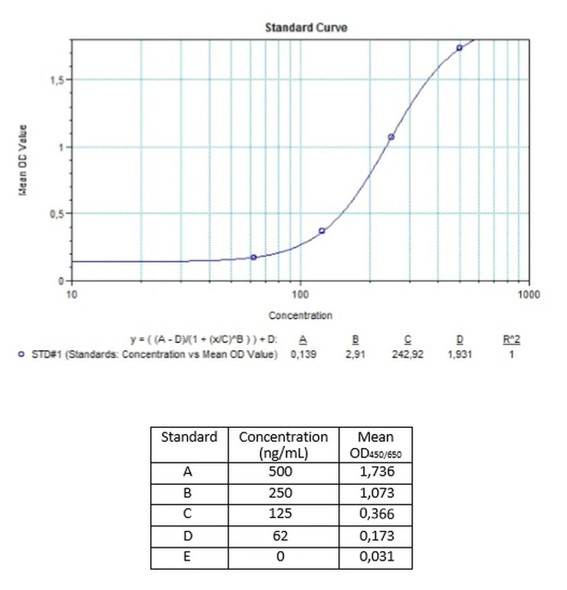
7 x 1 mL | AIR Standards A-E, High Level Control, Low Level Control 500; 250; 125; 62; 0 ng/mL |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Confirmation Reagent |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Film |
2 x 1 | Semi-Log Graph Paper |
Anti-Infliximab ADA Quantitative ELISA Protocol
| Steps | Protocol |
1 | QUANTITATIVE ELISA TEST FORMAT Wells: A1: Negative Control |
2 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
3 | Remove adhesive film. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
4 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
5 | Cover the plate with adhesive film. Incubate 60 min at room temperature (18- 25°C). |
6 | Remove adhesive film. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
7 | Pipette 100 µL of TMB Substrate Solution into each well. |
8 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
9 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
10 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
REMSIMA is a trademark of CELLTRION, INC.