How to Choose a Secondary Antibody
The selection of a secondary antibody is a critical step in the experimental design of various immunodetection methods, including Western Blot, ELISA, immunohistochemistry (IHC), and immunofluorescence (IF). Secondary antibodies serve as crucial tools for the amplification of signal detection, enabling researchers to observe specific antigens with high sensitivity and specificity. This article provides a comprehensive guide on how to choose the right secondary antibody for your research, ensuring the success of your immunodetection assays.
Understanding Secondary Antibodies
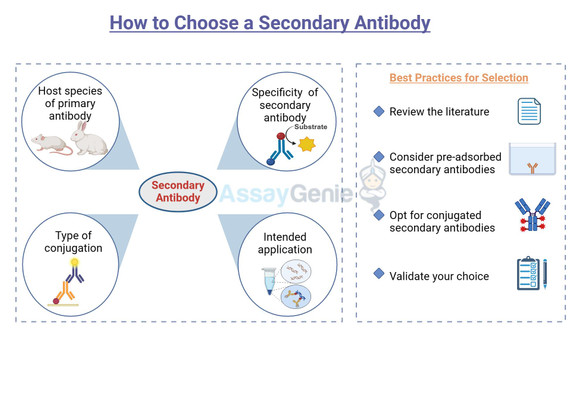
Secondary antibodies are antibodies that bind to the primary antibodies, which are directly bound to the target antigen. They are typically conjugated with a detection marker, such as an enzyme or a fluorophore, which facilitates the visualization of the antigen-antibody complex. The choice of secondary antibody depends on several factors, including the host species of the primary antibody, the specificity of the secondary antibody, the type of conjugation, and the intended application.
Host Species and Cross-Reactivity
One of the first considerations in choosing a secondary antibody is the host species of the primary antibody. It is crucial to select a secondary antibody that is raised against the species of the primary antibody. For instance, if your primary antibody is raised in rabbits, you should choose an anti-rabbit secondary antibody.
Cross-reactivity is another important factor. Ideally, the secondary antibody should not cross-react with endogenous immunoglobulins from the species of the sample tissue or cells. This consideration is especially important in IHC and IF applications, where endogenous immunoglobulins can lead to non-specific staining.
Conjugation and Detection Methods
The choice of conjugation is dictated by the detection method. For colorimetric detection, enzymes like horseradish peroxidase (HRP) or alkaline phosphatase (AP) are commonly used. For fluorescence detection, fluorophores such as FITC, TRITC, or Alexa Fluor dyes are preferred. The selection depends on the available detection equipment, such as fluorescence microscopes or flow cytometers, and the need for multiplexing, which requires fluorophores with non-overlapping emission spectra.
Affinity and Specificity
Applications and Experimental Conditions
Best Practices for Selection
Conclusion
References
- Green, N. M. (1990). Avidin and streptavidin. Methods in Enzymology, 184, 51-67.
- Harlow, E., & Lane, D. (1988). Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press.
- Hermanson, G. T. (2013). Bioconjugate Techniques (3rd ed.). Academic Press.
- Atha, D. H., Manne, U., Grizzle, W. E., Wagner, P. D., Srivastava, S., & Reipa, V. (2010). Standards for immunohistochemical imaging: A protein reference device for biomarker quantitation. Journal of Histochemistry & Cytochemistry, 58(11), 1005-1014.
- Nerenberg, S. T., & Peetoom, F. (1970). Use of Immunoelectrophoresis and Immunodiffusion in Clinical Medicine. CRC Critical Reviews in Clinical Laboratory Sciences, 1(2), 303-350.
- Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual (3rd ed.). Cold Spring Harbor Laboratory Press.
- Towbin, H., Staehelin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America, 76(9), 4350-4354.
- Chang, C. J., Yang, Y. H., Liang, Y. C., Chiu, C. J., Chu, K. H., Chou, H. N., & Chiang, B. L. (2011). A novel phycobiliprotein alleviates allergic airway inflammation by modulating immune responses. American journal of respiratory and critical care medicine, 183(1), 15-25.
Written by Tehreem Ali
Tehreem Ali completed her MS in Bioinformatics and conducted her research work at the IOMM lab at GCUF, Pakistan.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025