The Ubiquitin Proteasome System in Neurodegenerative disease
By Sinead Kinsella PhD
The Ubiquitin Proteasome System (UPS)
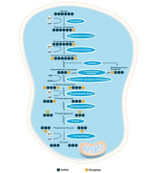
The Ubiquitin Proteasome System (UPS) is a regulatory machinery for protein turnover in the cell (Pickart, 2001), and strict regulation of the balance between protein synthesis and degradation is essential for cellular homeostasis (Gaczynska et al., 2001). The UPS is composed of the ubiquitination system, which governs the labelling of proteins for degradation, and the proteasome, which carries out protein degradation (Jansen et al., 2014). Ubiquitin degradation is a highly regulated process with specific targeting of the protein with ubiquitin molecules prior to degradation by the 26S proteasome, also termed the constitutive proteasome (Baumeister et al., 1998). The proteasome also contains a 19S proteasomal subunit which regulates unfolding of the ubiquitinated protein and guides it to the 26S core for degradation, a process requiring ATP (Smith et al., 2005).
The conversion of the UPS to the Immunoproteasome in the CNS
UPS dysfunction has been demonstrated to occur in several proteinopathies and is proposed to contribute to the accumulation of intracellular misfolded protein aggregates. The increased glial reactivity seen in neuroinflammation, termed reactive gliosis, induces a conformational change in the proteasomal subunit composition and the formation of the immunoproteasome (20i) (Jansen et al., 2014). The immunoproteasome is differentiated from the constitutive proteasome by the conversion of the constitutive catalytic subunits β1, β2 and β5 to the immuno-subunits β1i, β2i and β5i (Akiyama et al., 1994). A decrease in the constitutive proteasome and increase in the immunoproteasome correlates with the inflammatory response mediated by glial cells in neurodegenerative disease (Mendonca et al., 2006, Cheroni et al., 2005).

window.SHOGUN_IMAGE_ELEMENTS = window.SHOGUN_IMAGE_ELEMENTS || new Array(); window.SHOGUN_IMAGE_ELEMENTS.push({ hoverImage: '', uuid: 's-ec3613a9-f68b-4480-b68e-28d6450b4d86' })
Figure 1: Conversion of the Ubiquitin Proteasome System to the Immunoproteasome. Upon inflammation of cellular stress the constitutive proteasome converts to the immunoproteasome, characterized by the substitution of the β1, β2 and β5 to the immuno-subunits β1i, β2i and β5. This conformational change leads to dysregulated proteasomal degradation and contributes to the accumulation of protein aggregates observed in neurodegenerative pathologies.
The Immunoproteasome in disease
Impairment of the ubiquitin proteasome system is implicated in neurodegenerative disease, with misfolded proteins forming insoluble inclusions as they co-localize with ubiquitin proteasome subunits (Zheng et al., 2014, Ross and Poirier, 2004), leading to increased levels of neuronal misfolded protein aggregates (Jansen et al., 2014, Cheroni et al., 2005, Mori et al., 1987). Interestingly, examination of the UPS in the CNS overexpressing misfolded proteins identified lower UPS activity in neurons compared with glia, which is proposed to account for the higher levels and lack of clearance of protein aggregates observed in neurons (Jansen et al., 2014, Tydlacka et al., 2008). Intracellular aggregation of mutant SOD1 in microglia has been shown to contribute to ALS disease progression (Henkel et al., 2009, Puttaparthi and Elliott, 2005), with both K48-linked and K63-linked ubiquitin chains associated with SOD1G93A aggregate pathology (Tan et al., 2008).
Additionally, in ALS the accumulation of intracellular SOD1G93A aggregates leads to modifications in proteasomal function in an attempt to be cleared and degraded by the ubiquitin proteasome mediated pathway forming what has been called the ‘’immunoproteasome’’ (Tydlacka et al., 2008, Bence et al., 2001). Initial induction of the immunoproteasome is suggested to be a protective mechanism, however it is reported to be ineffective in the clearance of intracellular mutated protein aggregates and is believed to exacerbate pathogenesis (Puttaparthi and Elliott, 2005). In mutant SOD1-associated ALS, M1 microglia have an increased immunoproteasome and increased pro-inflammatory activation, resulting from both intracellular and extracellular mutant SOD1 stimulation, as extracellular SOD1G93A has been demonstrated to be a ligand for TLR2 and TLR4 (Zhao et al., 2010), and intracellular SOD1G93A aggregates induce the formation of the immunoproteasome (Henkel et al., 2009).
Intracellular aggregates of misfolded proteins induce a chronic self-propagating microglial inflammatory response (Orre et al., 2013). Additionally, mutations in Ubiquilin 2 (Ubqln2) are found in to be linked to ALS pathogenesis (Deng et al., 2011), and are proposed to inhibit the targeting of ubiquitinated proteins to the proteasome (Chang and Monteiro, 2015), enhancing aggregate accumulation. Specific inhibitors of the induced immunoproteasome subunits has been proposed as an effective therapy targeting glial cell activation in neurodegeneration (Miller et al., 2013). Furthermore, studies have found that ubiquitin co-localizes with mutant SOD1 aggregates in ALS (Stieber et al., 2000), and reduced proteasomal activity contributes to the lack of clearance of SOD1G93A (Hoffman et al., 1996). In addition, an atypical form of ubiquitination has been identified in Parkinson’s disease as co-localization of α-synuclein aggregates with ubiquitin induce inclusion formation (Zucchelli et al., 2010).
Targeting the immunoproteasome by alleviating the downregulated neuronal proteasome and upregulated the glial cell proteasome may ameliorate the polarization of microglia to the M1 state, thereby providing an alternative therapeutic strategy towards attenuating the chronic inflammatory response driving disease progression in neurodegeneration.
References:
AKIYAMA, K., KAGAWA, S., TAMURA, T., SHIMBARA, N., TAKASHINA, M., KRISTENSEN, P., HENDIL, K.B., TANAKA, K., ICHIHARA, A. 1994. Replacement of proteasome subunits X and Y by LMP7 and LMP2 induced by interferon-gamma for acquirement of the functional diversity responsible for antigen processing. FEBS Lett. 343(1):85-8.
BAUMEISTER, W., WALTZ, J., ZUHL, F., SEEMULLER, E. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998 Feb 6;92(3):367-80.
BENCE, N. F., SAMPAT, R. M. & KOPITO, R. R. 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science, 292, 1552-5.
CHANG, L. & MONTEIRO, M. J. 2015. Defective Proteasome Delivery of Polyubiquitinated Proteins by Ubiquilin-2 Proteins Containing ALS Mutations. PLoS One, 10, e0130162.
CHERONI, C., PEVIANI, M., CASCIO, P., DEBIASI, S., MONTI, C. & BENDOTTI, C. 2005. Accumulation of human SOD1 and ubiquitinated deposits in the spinal cord of SOD1G93A mice during motor neuron disease progression correlates with a decrease of proteasome. Neurobiol Dis, 18, 509-22.
DENG, H. X., CHEN, W., HONG, S. T., BOYCOTT, K. M., GORRIE, G. H., SIDDIQUE, N., YANG, Y., FECTO, F., SHI, Y., ZHAI, H., JIANG, H., HIRANO, M., RAMPERSAUD, E., JANSEN, G. H., DONKERVOORT, S., BIGIO, E. H., BROOKS, B. R., AJROUD, K., SUFIT, R. L., HAINES, J. L., MUGNAINI, E., PERICAK-VANCE, M. A. & SIDDIQUE, T. 2011. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature, 477, 211-5.
GACZYNSKA, M., OSMULSKI, P. A. & WARD, W. F. 2001. Caretaker or undertaker? The role of the proteasome in aging. Mech Ageing Dev, 122, 235-54.
HENKEL, J. S., BEERS, D. R., ZHAO, W. & APPEL, S. H. 2009. Microglia in ALS: the good, the bad, and the resting. J Neuroimmune Pharmacol, 4, 389-98.
HOFFMAN, E. K., WILCOX, H. M., SCOTT, R. W. & SIMAN, R. 1996. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J Neurol Sci, 139, 15-20.
JANSEN, A. H., REITS, E. A. & HOL, E. M. 2014. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci, 7, 73.
MENDONCA, D. M., CHIMELLI, L. & MARTINEZ, A. M. 2006. Expression of ubiquitin and proteasome in motorneurons and astrocytes of spinal cords from patients with amyotrophic lateral sclerosis. Neurosci Lett, 404, 315-9.
MILLER, Z., AO, L., KIM, K. B. & LEE, W. 2013. Inhibitors of the immunoproteasome: current status and future directions. Curr Pharm Des, 19, 4140-51.
MORI, H., KONDO, J. & IHARA, Y. 1987. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science, 235, 1641-4.
ORRE, M., KAMPHUIS, W., DOOVES, S., KOOIJMAN, L., CHAN, E. T., KIRK, C. J., DIMAYUGA SMITH, V., KOOT, S., MAMBER, C., JANSEN, A. H., OVAA, H. & HOL, E. M. 2013. Reactive glia show increased immunoproteasome activity in Alzheimer’s disease. Brain, 136, 1415-31.
OSAKA, M., ITO, D., YAGI, T., NIHEI, Y. & SUZUKI, N. 2015. Evidence of a link between ubiquilin 2 and optineurin in amyotrophic lateral sclerosis. Hum Mol Genet, 24, 1617-29.
PICKART, C. M. 2001. Mechanisms underlying ubiquitination. Annu Rev Biochem, 70, 503-33.
PUTTAPARTHI, K. & ELLIOTT, J. L. 2005. Non-neuronal induction of immunoproteasome subunits in an ALS model: possible mediation by cytokines. Exp Neurol, 196, 441-51.
ROSS, C. A. & POIRIER, M. A. 2004. Protein aggregation and neurodegenerative disease. Nat Med, 10 Suppl, S10-7.
SMITH, D,M., KAFRI, G., CHENG, Y., NG, D., WALZ, T., GOLDBERG, AL. 2005. ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol Cell. 20(5):687-98.
STIEBER, A., GONATAS, J. O. & GONATAS, N. K. 2000. Aggregation of ubiquitin and a mutant ALS-linked SOD1 protein correlate with disease progression and fragmentation of the Golgi apparatus. J Neurol Sci, 173, 53-62.
TAN, J. M., WONG, E. S., KIRKPATRICK, D. S., PLETNIKOVA, O., KO, H. S., TAY, S. P., HO, M. W., TRONCOSO, J., GYGI, S. P., LEE, M. K., DAWSON, V. L., DAWSON, T. M. & LIM, K. L. 2008. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum Mol Genet, 17, 431-9.
TYDLACKA, S., WANG, C. E., WANG, X., LI, S. & LI, X. J. 2008. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J Neurosci, 28, 13285-95.
ZHAO, W., BEERS, D. R., HENKEL, J. S., ZHANG, W., URUSHITANI, M., JULIEN, J. P. & APPEL, S. H. 2010. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia, 58, 231-43.
ZHENG, C., GEETHA, T. & BABU, J. R. 2014. Failure of ubiquitin proteasome system: risk for neurodegenerative diseases. Neurodegener Dis, 14, 161-75.
ZUCCHELLI, S., CODRICH, M., MARCUZZI, F., PINTO, M., VILOTTI, S., BIAGIOLI, M., FERRER, I. & GUSTINCICH, S. 2010. TRAF6 promotes atypical ubiquitination of mutant DJ-1 and alpha-synuclein and is localized to Lewy bodies in sporadic Parkinson’s disease brains. Hum Mol Genet, 19, 3759-70.
Recent Posts
-
Biological Role of GLP-1
Glucagon-like peptide-1 (GLP-1) is a critical hormone in the regulation of glucose met …20th Jun 2024 -
Th17 Cell Differentiation: Insights into Immunological Dynamics
Th17 cells, a subset of T helper cells characterized by their production of interleukin-17 (IL- …25th May 2024 -
Assay Genie New Asian Distributor May 2024
Dublin, Ireland — May 20th 2024 — Assay Genie, a leading supplier of ELISA Kits, Ant …19th May 2024