Understanding T Cell Fatigue: Implications for Immune Function and Therapeutic Interventions
T cells are central players in adaptive immunity, responsible for recognizing and eliminating infected or aberrant cells. Upon encountering antigens, T cells undergo clonal expansion and differentiation into effector or memory cells, executing various functions to eliminate the threat. However, in scenarios of persistent antigen exposure, such as chronic infections or cancer, T cells can become functionally exhausted, leading to compromised immune responses. This phenomenon, known as T cell fatigue or exhaustion, has garnered significant attention due to its implications for immune function and therapeutic interventions.
Mechanisms of T Cell Fatigue:
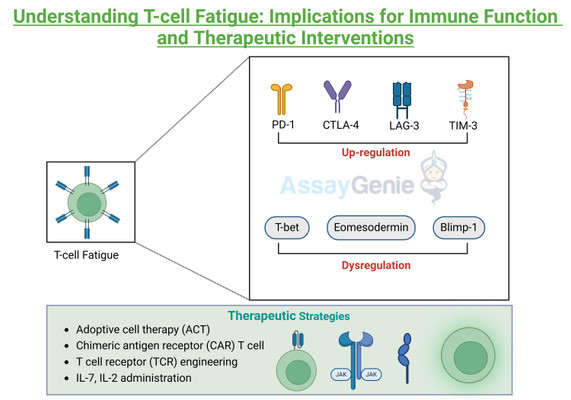
T cell fatigue is characterized by a progressive loss of effector function and proliferation capacity, accompanied by sustained expression of inhibitory receptors. The molecular mechanisms underlying T cell fatigue involve intricate interplays between various signaling pathways and transcriptional regulators. Chronic antigen stimulation leads to the upregulation of inhibitory receptors, such as programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3), and T cell immunoglobulin and mucin-domain containing-3 (TIM-3). Engagement of these receptors with their ligands, often overexpressed on target cells or antigen-presenting cells, initiates inhibitory signaling cascades, dampening T cell activation and effector functions. Furthermore, transcriptional profiling of exhausted T cells reveals dysregulated expression of key transcription factors, such as T-bet, Eomesodermin (Eomes), and Blimp-1, which orchestrate effector and exhaustion programs. Additionally, metabolic reprogramming, characterized by impaired mitochondrial function and glycolysis, contributes to T cell dysfunction during exhaustion.
Schematic Representation of Mechanism of Tcell fatigue
Consequences of T Cell Fatigue:
The consequences of T cell fatigue extend beyond impaired pathogen clearance, impacting overall immune homeostasis and susceptibility to infections and cancer. In chronic viral infections, such as human immunodeficiency virus (HIV) and hepatitis B virus (HBV), T cell exhaustion facilitates viral persistence and disease progression. Similarly, in the context of cancer, exhausted T cells exhibit reduced cytotoxicity and cytokine production, enabling tumor evasion from immune surveillance. Moreover, T cell fatigue compromises the formation of memory T cells, impairing long-term immune protection against recurring infections.
Therapeutic Strategies for T Cell Fatigue:
Understanding the molecular mechanisms underlying T cell fatigue has paved the way for the development of therapeutic strategies aimed at rejuvenating exhausted T cells and restoring immune function. Immune checkpoint blockade (ICB) therapies, targeting inhibitory receptors such as PD-1, CTLA-4, and LAG-3, have revolutionized cancer treatment by unleashing T cell-mediated anti-tumor responses. Similarly, interventions targeting metabolic pathways, such as enhancing mitochondrial function or modulating glycolysis, hold promise for reversing T cell exhaustion. Furthermore, adoptive cell therapy (ACT) approaches, including chimeric antigen receptor (CAR) T cells and T cell receptor (TCR) engineering, offer personalized strategies to augment T cell responses against cancer and chronic infections. Additionally, cytokine-based therapies, such as interleukin-2 (IL-2) or interleukin-7 (IL-7) administration, can enhance T cell proliferation and effector functions, potentially overcoming exhaustion-associated defects.
Conclusion:
References:
- Wherry, E. J., & Kurachi, M. (2015). Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology, 15(8), 486–499.
- Pauken, K. E., & Wherry, E. J. (2015). SnapShot: T Cell Exhaustion. Cell, 163(4), 1038–1038.e1.
- Blank, C. U., & Haining, W. N. (2019). Contrasting T Cell Dysfunction in Cancer and Chronic Viral Infection: Implications for Cancer Immunotherapy. Immunological Reviews, 276(1), 1–7.
- McLane, L. M., & Abdel-Hakeem, M. S. (2019). Understanding the Biology of Exhausted CD8 T Cells: Lessons for AIDS, Cancer, and Chronic Infections. Journal of Infectious Diseases, 220(3), 174–185.
- Khan, O., Giles, J. R., McDonald, S., Manne, S., Ngiow, S. F., Patel, K. P., Werner, M. T., Huang, A. C., Alexander, K. A., Wu, J. E., Attanasio, J., Yan, P., George, S. M., Bengsch, B., Staupe, R. P., Donahue, G., Xu, W., Amaravadi, R. K., Xu, X., … Wherry, E. J. (2019). TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature, 571(7764), 211–218.
- Paley, M. A., Kroy, D. C., Odorizzi, P. M., Johnnidis, J. B., Dolfi, D. V., Barnett, B. E., Bikoff, E. K., Robertson, E. J., Lauer, G. M., Reiner, S. L., & Wherry, E. J. (2012). Progenitor and Terminal Subsets of CD8+ T Cells Cooperate to Contain Chronic Viral Infection. Science, 338(6111), 1220–1225.
- Im, S. J., Hashimoto, M., Gerner, M. Y., Lee, J., Kissick, H. T., Burger, M. C., Shan, Q., Hale, J. S., Lee, J., Nasti, T. H., Sharpe, A. H., Freeman, G. J., Germain, R. N., Nakaya, H. I., Xue, H.-H., & Ahmed, R. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature, 537(7620), 417–421.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026