8-OHdG / 8-Hydroxydeoxyguanosine ELISA Kit
- SKU:
- UNFI0029
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Sensitivity:
- 0.281 ng/ml
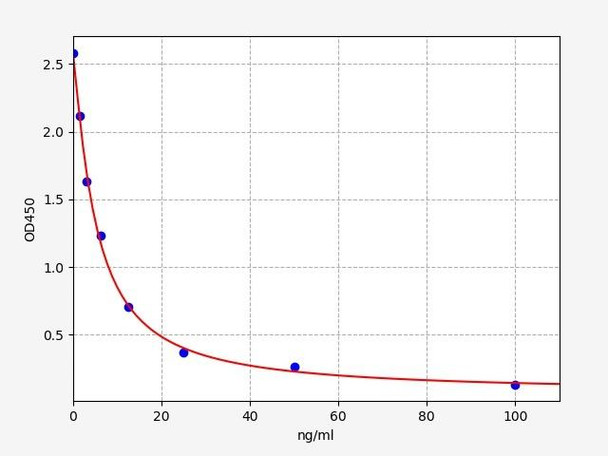
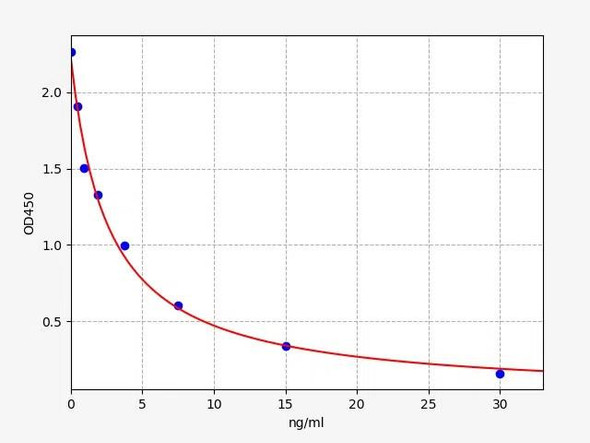
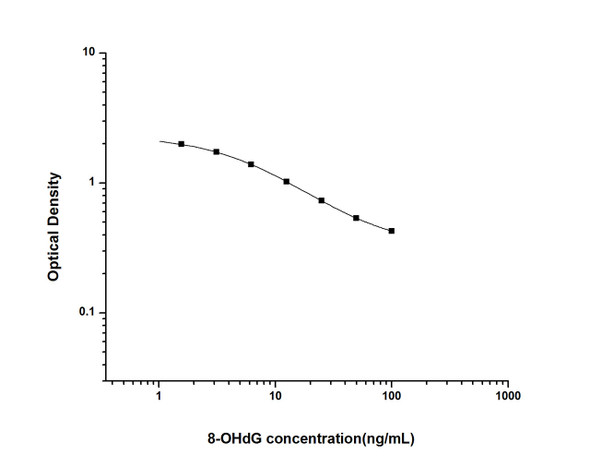
- Range:
- 0.468-30 ng/ml
- ELISA Type:
- Competitive
- Synonyms:
- 8-OHdG, 8-Hydroxydeoxyguanosine
- Reactivity:
- Universal
Description
8-OHdG ELISA Kit
Urinary 8-OHdG is a biomarker for oxidative damage, cancers, diabetes and heart disease. The Assay Genie 8-OHdG ELISA Kit has been validated in urine and comes with extensive validation data including Spike-recovery, Range and Linearity. Furthermore the 8-OHdG ELISA is one of the most sensiteve kits on the market to date, allowing researchers to forgo the laborious separation and isolation steps of HPLC and detect low levels of 8-OHdG across any species.
Key Features
| Save Time | Pre-coated 96 well plate | |
| Quick Start | Kit includes all necessary reagents | |
| Publication Ready | Reproducible and reliable results |
Overview
| Product Name: | 8-OHdG (8-Hydroxydeoxyguanosine) ELISA Kit |
| Product Code: | UNFI0029 |
| Size: | 96 Assays |
| Target: | 8-OHdG |
| Alias: | 8-OHdG, 8-Hydroxydeoxyguanosine |
| Reactivity: | Universal |
| Detection Method: | Competitive ELISA, Coated with Antibody |
| Sensitivity: | 0.281 ng/ml |
| Range: | 0.468-30 ng/ml |
| Storage: | 4°C for 6 months |
| Note: | For Research Use Only |
Additional Information
| New Column | ||||||||||||||||||||||
| Recovery | Matrices listed below were spiked with certain level of 8-OHdG and the recovery rates were calculated by comparing the measured value to the expected amount of 8-OHdG in samples.
| |||||||||||||||||||||
| Linearity: | The linearity of the kit was assayed by testing samples spiked with appropriate concentration of [ANALYTE] and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| |||||||||||||||||||||
| Intra-Assay: | CV <8% | |||||||||||||||||||||
| Inter-Assay: | CV <10% |
Protocol
*Note: Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Equilibrate the TMB substrate for at least 30 min at 37°C beforeuse. When diluting samples and reagents, they must be mixed completely andevenly. It is recommended to plot a standard curve for each test.
| Step | Procedure |
| 1. | Set standard, test sample and control (zero) wells on the pre-coatedplate respectively, and then, record their positions. It isrecommended to measure each standard and sample in duplicate. Washplate 2 times before adding standard, sample and control (zero) wells! |
| 2. | Add Sample and Biotin-detection antibody: Add 50µL of Standard, Blank or Sample per well. The blankwell is added with Sample Dilution Buffer. Immediately add 50 µL of biotin-labelled antibody workingsolution to each well. Cover with the plate sealer provided. Gently tap the plate to ensure thoroughmixing. Incubate for 45 minutes at 37°C. (Solutions are added to the bottom of micro-ELISA platewell, avoid touching plate walls and foaming). |
| 3. | Wash: Aspirate each well and wash, repeating the process three timesWash by filling each well with Wash Buffer (approximately 350µL)using a squirt bottle, multi-channel pipette, manifold dispenser orautomated washer. Complete removal of liquid at each step is essentialto good performance. After the last wash, remove any remaining WashBuffer by aspirating or decanting. Invert the plate and pat it againstthick clean absorbent paper. |
| 4. | HRP-Streptavidin Conjugate(SABC): Add 100µL of SABC workingsolution to each well. Cover with a new Plate sealer. Incubate for30minutes at 37°C. |
| 5. | Wash: Repeat the aspiration/wash process for five times. |
| 6. | TMB Substrate: Add 90µL of TMB Substrate to each well. Coverwith a new Plate sealer. Incubate for about 10-20 minutes at 37°C.Protect from light. The reaction time can be shortened or extendedaccording to the actual color change, but not more than 30minutes.When apparent gradient appeared in standard wells, you can terminatethe reaction. |
| 7. | Stop: Add 50µL of Stop Solution to each well. Color turn toyellow immediately. The adding order of stop solution should be as thesame as the substrate solution. |
| 8. | OD Measurement: Determine the optical density (OD Value) of each wellat once, using a microplate reader set to 450 nm. You should open themicroplate reader ahead, preheat the instrument, and set the testing parameters. |
Sample Type
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |
8-OHdG Background
8-OHdG (8-Hydroxydeoxyguanosine) is a modified nucleoside derived from the oxidation of guanine, one of the four nitrogenous bases found in DNA. It is formed as a result of oxidative stress-induced damage to DNA caused by reactive oxygen species (ROS) and other free radicals. ROS are byproducts of normal cellular metabolism, but their excessive production or insufficient elimination can lead to oxidative stress, which can damage DNA, proteins, and lipids. Measuring 8-OHdG levels provides valuable insights into the extent of oxidative damage to DNA and serves as a marker for evaluating the effectiveness of antioxidant therapies, monitoring disease progression, and assessing the impact of environmental factors on cellular health.
8-OHdG FAQs
What is the 8-OHdG ELISA Kit?
An 8-OHdG / 8-Hydroxydeoxyguanosine ELISA Kit is designed to measure the levels of 8-OHdG (8-Hydroxydeoxyguanosine) in various biological samples. This ELISA (Enzyme-Linked Immunosorbent Assay) kit utilizes specific antibodies to detect and quantify the presence of 8-OHdG, a biomarker associated with oxidative stress and DNA damage.
What are the advantages of using the 8-OHdG ELISA Kit?
The 8-OHdG ELISA Kit offers several advantages. It provides a sensitive and specific method for quantifying 8-OHdG levels, allowing researchers to assess oxidative stress and DNA damage accurately. The kit comes with all the necessary reagents and detailed instructions, making it convenient and user-friendly. It also enables high-throughput analysis, allowing for the processing of multiple samples simultaneously.
Where can I find more information about the 8-OHdG ELISA Kit?
For more detailed information about the 8-OHdG ELISA Kit, including technical specifications, performance characteristics, and ordering details, please refer to the product brochure or contact our customer support team. We are here to assist you with any inquiries you may have.