A mechanopharmacology approach to overcome chemoresistance in pancreatic cancer
Mechanical forces are common in biological systems and dominate numerous perturbations that are relevant to human physiology and disease. Mechanobiology is a new field that evaluates the effects of physical forces on cell behavior, cell/tissue morphogenesis and diseases such as cancer. Most recently this field has matured in a way that it appears feasible to apply this approach for the identification of both disease-related signaling defects and of key determinants of chemoresistance.
As a member of a group of investigators led by Elisa Giovannetti at the VU University Medical Center in Amsterdam (The Netherlands) and at Cancer Pharmacology lab, AIRC Start-Up Unit, University of Pisa (Italy), my studies are aimed to implement personalized treatments into the clinic and clarify resistance mechanisms to conventional and novel anticancer drugs, with particular attention to pancreatic cancer.
In the last years a list of celebrities who were diagnosed and died of pancreatic cancer (Apple CEO and co-founder Steve Jobs, opera singer Luciano Pavarotti, actor Patrick Swayze) turning the spotlight on this extremely chemoresistant and lethal disease.
Thinking outside the box, and in collaboration with the Schmidt lab, at Leiden Institute of Physics, we aim to develop a new research field with potential strong implications in pancreatic cancer treatment: mechanopharmacology. The Schmidt lab has a longstanding tradition in investigating the interplay between mechanical cues and cellular response at (single-)molecular and cellular level. Unique super-resolution microscopy setup for cell-generated force measurements on micropillar arrays and cell-stretcher instruments with optical access have been recently developed for mechanobiological studies.
Our collaborative research with Dr. Coppola (awarded by a prestigious AXA Fellowships at the Schmidt lab) represents a new way of looking into mechanical interplay between tumor and stroma that might provide the missing link between genetics and the influence of the tumor microenvironment on pancreatic cancer chemoresistance. Therefore, we propose to unravel the effects of mechanical cues on chemoresistance in a multidisciplinary study in which expertise from pharmacology, molecular/cell biology and physics is combined with clinical data.
Pancreatic Cancer Chemoresistance
Pancreatic cancer is a devastating malignant disease, exhibiting one of the poorest prognoses of all solid tumors. Its very aggressive nature and the early metastatic behavior frequently impede the potentially curative surgical resection. Chemotherapy is, therefore, a crucial component in the treatment of unresectable (metastatic or locally-advanced) pancreatic cancer patients. However, the two most successful combination chemotherapeutic protocols [i.e. FOLFIRINOX (a combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin) and gemcitabine/nab-paclitaxel, resulted in modest survival benefits < 1 year] which are unfortunately nullified by the significant untoward toxicity and a compromised quality of life for most PDAC patient. Cancer chemoresistance can occur by multiple mechanisms.
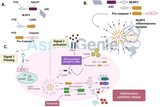
Pancreatic cancer chemoresistant phenotype has been historically associated with genetic factors. Major biomedical research efforts were concentrated that resulted in the identification of subtypes characterized by specific genetic lesions and gene expression signatures that suggest important biological differences. However, the increased knowledge of the underlying genetics of PDAC has not yet been translated to the identification of “actionable” targets. Apart from these genetic factors, desmoplasia, a fibrotic mass including cancer-associated fibroblasts and self-sustaining extracellular matrix (ECM) and tumor microenvironment have been recognized as key contributors to pancreatic cancer chemoresistance. This led to the development of treatments towards tumor stroma, which have so far failed to meet expectations. These unsatisfactory clinical results suggest that an important link between genetics and the influence of tumor microenvironment on chemoresistance remains to be elucidated. In our review “A mechanopharmacology approach to overcome chemoresistance in pancreatic cancer”, we describe the current knowledge regarding pancreatic cancer chemoresistance and the unsuccessful (pre)-clinical attempts to enhance the response to chemotherapeutics used in the clinical routine. From a mechanobiology perspective, we then elucidate the bidirectional interplay between drug action/resistance and mechanics, under the context of the highly genomically unstable landscape of pancreatic cancer.
Drug Resistance and Pancreatic Cancer
Recent studies demonstrated the pivotal role of several ECM components in drug resistance and cancer progression. Pancreatic cancer cells attached to collagen (type I and type IV) and fibronection (FN) showed a decreased sensitivity to chemotherapeutic treatment, while the malignant phenotype of pancreatic cancer cell lines has been correlated to the α2β1 integrin-mediated adhesion to type I collagen. In addition, FN exerts a prosurvival effect on cancer cells which is mediated by FAK dependent activation of the PI3K/AKT/mTOR pathway. FN-mediated activation of FAK starts cell proliferation through the recruitment of SH2-binding proteins such as Src and Grb2, which directly trigger the Ras pathway. Most recently, experimental mouse models revealed a unique, highly rigid, matricellular-stromal phenotype linked specifically to the quasi-mesenchymal PDAC subtype, which resulted in reduced epithelial TGF-β signaling as well as elevated β1-integrin and cell contractility. This latter groundbreaking study, as being largely based on genetics, however, failed to identify the role of mechanobiology in the chemosensitivity/resistance of pancreatic cancer, and further studies are warranted.
Our review provides a comprehensive overview of the key players in pancreatic cancer chemoresistance through a mechanobiology aspect, and discuss novel experimental tools such as elastic micropillar arrays that could provide fresh insights for the development of mechanobiology-targeted therapeutic approaches to overcome anticancer drug resistance in pancreatic cancer. In particular, we adopted the term ‘mechanopharmacology’ that has been recently introduced by Krishnan and colleagues to define a new and wider conceptual field, that aims at investigating the impact of cell and tissue mechanics on pharmacological responsiveness, and its application to mechanistic investigations and drug screening. Mechanopharmacology requires the combination of innovative methodologies and concepts in biophysics, engineering, and medicine. It is our strong belief that this new field of research could be successful at the discovery of novel drug targets and antitumor agents to combat pancreatic cancer.
Ilaria Carnevale, PhD
Postdoc Scientific Researcher
VU University Medical Center in Amsterdam (The Netherlands) (https://www.vumc.com/branch/Medical-Oncology/3883764/3851997)
Cancer Pharmacology Lab, University of Pisa (Italy) (http://www.cancerpharmacology.org/our-team/elisa-giovannetti/)
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024