Bone Morphogenetic Proteins (BMP) – Review
Bone Morphogenetic Proteins (BMP) were first discovered in the 1960s by Dr.Marshall Urist, an orthopaedic surgeon at UCLA (Urist 1965). BMPs are classically associated with their roles in limb development, induction of cartilage and bone growth. However, it has been since clarified that Bone Morphogenetic proteins (BMPs) are involved in many more diverse biological processes, such as stem cell and organ formation, muscle development, iron metabolism, vascular biology and cancer (D. P. Brazil et al.2015).
The BMPs belong to the TGF-β superfamily and are glycosylated, extracellular matrix-associated molecules. The constituents of the BMP signalling pathway have also been implicated in determining the rate of progression of a number of diseases (Boers et al. 2006; Costello et al. 2010; Roxburgh et al. 2006). Bone Morphogenetic Protein (BMP) sequences are similar within and between species. Phylogenetic analysis of human BMP sequences show that there are conserved and similar residues across the sequences and this in-turn translates to conserved moieties facilitating dimerization, receptor binding and antagonist binding (Ducy & Karsenty 2000).
Molecular Mechanism of Bone Morphogenetic Protein (BMP) Action
Bone Morphogenetic Proteins (BMPs) are secreted as large, immature proproteins with a latency domain that prevents dimerization. This N-terminal prodomain of BMP is cleaved by proteases at Arg-X-X-Arg recognition sequences allowing dimerization (Aono et al. 1995). The mature form is approximately a third of the total length of the precursor and contains consensus sequences for the eight amino acid disulfide bond cysteine knot – C43-X-G-X-C47, C111-X-C113 and C14/C79 (Avsian-Kretchmer & Hsueh 2004). The secreted BMPs and their antagonists are limited in their diffusion and restricted in their action to neighbouring cells. They are thought to associate with the ECM (Extracellular Matrix). Glycosylation is likely to facilitate their interaction with the ECM (Miyazaki et al. 2008; Hang et al. 2014).
Activated BMP
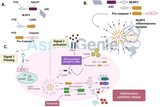
Active, cleaved Bone Morphogenetic Protein (BMP) monomers associate to generally form homodimers (and in some cases heterodimers). The homodimeric BMPs bind specific type I and type II receptors at the cell surface membrane. There are five type I BMP receptors: activin-like kinase (ALK) ALK1 (Acvrl1), ALK2 (ActRI), ALK3 (BMPR1a), ALK4 (ActRIb), ALK6 (BMPRIb) and three type II BMP receptors: BMPRII, ActRIIa, and ActRII (Nohe et al. 2004). BMP signalling is initiated by the interaction of BMP to specific high-affinity type I and type II serine threonine kinase transmembrane receptors. This hexameric complex consists of two type I receptors, two type II receptors and a BMP ligand dimer. Upon association, the type II receptor transphosphorylates the type I receptor at GS (glycine, serine) rich regions thereby activating type I receptor kinase activity.
The activation of the type I receptor triggers binding and phosphorylation of receptor-associated SMAD 1/5/8. This interaction is facilitated by molecular scaffolds called SMAD Anchor for Receptor Activation (SARA) and Endofin. These act in similar ways by binding BMP-specific R-SMADs and promoting signal transduction by localizing early endosomes and facilitating transphosphorylation. The R-Smads bind to SMAD 4, which translocate and accumulate in the nucleus and are recruited to transcriptional complexes to mediate BMP-dependent gene transcription (Lo et al. 2002; Rider & Mulloy 2010; Walsh et al. 2010).
Bone Morphogenetic Protein Signalling
At a cellular level,Bone Morphogenetic Protein (BMP) signalling is regulated by the transcription of inhibitory SMADs (I-SMADs), such as SMAD 6 and SMAD 7. The I-SMADs bind directly to the BMP type I receptors and recruit SMAD specific E3 ubiquitin ligase (Smurf1). This leads to the competitive inhibition of R-SMAD binding to the type I receptor thereby preventing activation of phosphorylation by the receptor. Smurf1 interaction with the receptors leads to type I receptor ubiquitination and degradation (Ebisawa et al. 2001; Murakami et al. 2003; Suzuki et al. 2002). There is also additional regulation of BMP signalling by mechanisms such as cytosolic phosphatases, miRNA, methylation and control of BMP-mediated gene expression (D. Brazil et al. 2015).
At an extracellular level, a diverse range of BMP antagonists modulate the interaction between the cell surface receptors and the BMP ligands. BMP antagonists, such as Noggin, Crossveinless-2 (CV-2), Protein Related to DAN/Cerberus (PRDC) and Gremlin-1, are also secreted as proproteins. After proteolytic cleavage of their latency domain, the BMP-binding N-terminal domain is revealed. The antagonists are now mature and activate. The antagonists bind BMP there by occluding BMP ligand association to both types of BMP receptor (Avsian-Kretchmer & Hsueh 2004).
Bone Morphogenetic Protein Signalling in Disease
Bone Morphogenetic Protein (BMP) signalling plays a critical role in development and disease, which is exemplified by the extreme phenotypes in transgenic or knock-out mice lacking either BMPs, BMP receptors or BMP antagonists. Mice deficient in BMP-2 and -4 are nonviable due to heart defects (Zhang & Bradley 1996) and mice deficient in BMP-7 have malformed kidneys and die shortly after birth (A. Dudley et al. 1995).
Described in the following is a brief overview of mutations in BMP signalling that contribute to human disease. Such as fibroses, cancers and muscoskeletal defects. This is by no means a comprehensive guide but shows that each constitutive element of the signalling pathway can be affected and contributes to a disease phenotype. Many excellent reviews describe in greater detail the effects of aberrant BMP signalling in disease and also the effects of knockouts in model organisms (Walsh et al. 2010; D. Chen et al. 2004a; Wang et al. 2014; Bragdon et al. 2011; D. Brazil et al. 2015).
Role of Bone Morphogenetic Protein (BMP) in human disease
Mutations in Bone Morphogenetic Proteins (BMPs) have long been known to play major roles in many inherited diseases. The BMP signalling pathway itself has also become an important target for therapeutic development (D. Brazil et al. 2015). Mutations in BMP-5 are known to cause a range of skeletal defects in mice, including a short ear phenotype, a reduction in long bone width and overall lower body mass (Kingsley et al. 1992; Mikić et al. 1995) and mutations in BMP-11 are shown to cause chondrodysplasia in humans (Thomas et al. 1996).
Not only do mutations cause abnormal signalling and cause disease phenotypes, for example ectopic expression of BMP-4 was found in fibrodysplasia ossificans progressiva (FOP) patients. It is an extremely rare and disabling genetic disorder characterized by the congenital malformation of the great toes and by progressive heterotopic endochondral ossification in predictable anatomical patterns (Gannon et al. 1997; Xu et al. 2000). Over expression of the genes for BMP-2, BMP-3 and, more recently, BMP-6 have all been detected in prostate cancer cells (Harris et al. 1994; Darby et al. 2008).
Role of BMP receptor in disease
It has also been observed that both types of Bone Morphogenetic Protein (BMP) receptor can also contribute to defects if mutated. For example, a null mutation of BMPRIb in mice was found to cause sever appendicular skeletal defects (Yi et al. 2000). Overexpression of BMPR1a was also associated with carcinogenesis and malignancy of oral epithelium (Jin et al. 2001). A mutation in the gene expressing the type II receptor BMPRII was found by screening patients with familial primary pulmonary hypertension (Newman et al. 2001).
Role of BMP antagonist in human disease
Many BMP antagonists are associated with disease states including cancer, skeletal disorders and fibrosis of kidney, lung, liver, eye and heart. In cancers, BMP antagonists can sometimes play a pro or anti-tumorigenic role in vivo. For example, overexpression of Noggin promotes skin tumorigenesis via stimulation of the Wnt and Shh signalling pathways (Sharov et al. 2009). Overexpression of Gremlin-1 has also been detected in a range of human tumours including cervical cancer (Namkoong et al. 2006) and basal cell carcinoma (Sneddon et al. 2006).
Furthermore, mutations in Sclerostin and Noggin can result in a number of rare genetic skeletal disorders, such as Bone Dysplasia Sclerosteosis for Sclerostin (Brunkow et al. 2001), and Multiple Synostoses Syndrome 1 and Brachydactylyl Type B2 for Noggin (Brown et al. 2002; Gong et al. 1999; Lehmann et al. 2007). BMP antagonists are also associated with fibrosis. Increased expression of Gremlin-1 is also associated with the progression of diabetic retinopathy (Kane et al. 2005) and liver fibrosis, chronic hepatitis and liver cirrhosis (Boers et al. 2006; Guimei et al. 2012). Upregulation of Follistatin is also associated with liver fibrosis (Boers et al. 2006).
BMP structure, receptors and antagonists
BMP structure
The Bone Morphogenetic Protein (BMP) family share very similar ‘butterfly-like’ structures (except for BMP-1 which is not part of the BMP family). In general, the BMP dimer contains a cysteine-knot fold and the two subunits are convalently connected by a disulphide bond. Each monomer is made up of a two fingers, each finger formed by two pairs of anti-parallel β sheets stretched outward from the cysteine-knot core. The cysteine knot core contains seven disulfide bonds.
The curvature of the extended fingers creates a concave surface in which the α3 helix of the other subunit binds and stabilizes the dimer. The core of BMPs are characterized by the eight membered ring, made up of segments (BMP-7 numbering) of five (CEGEC71) and three (CGC138) residues which are linked by disulfide bonds between Cys-67 and Cys-136, and between Cys-71 and Cys-138. A third disulfide bond, between Cys-38 and Cys-104, penetrates through the middle of the ring, forming a ten membered cysteine knot.
BMP: receptor complexes
A BMP-2 ternary complex with ActII and BMPR1a gives great structural insight to BMP family: Bone Morphogenetic Protein (BMP) receptors interactions. The BMP mature dimer possesses binding surfaces that are termed the ‘wrist’ and ‘knuckle’ epitopes. The wrist epitope located at the cavity formed by the overlap of the two BMP subunits. The type I receptor interacts with both BMP subunits in the wrist epitope previously described as a ‘knob-into-hole’ motif (Kirsch et al. 2000).
BMP-2 and BMPR-I
In the case of BMP-2 and BMPR-Ia, the buried surface formed by the interaction of 25 BMPR1a residues with 28 BMP-2 residues (12 residues from one, 16 residues from the other) is 1217 A2. The interacting residues are generally hydrophobic, but in this case two binding pockets are formed. The first pocket involves Phe-49, Pro-50 of α-helix 2 of BMP-2 which interact with Ile-62, Phe-60 and Ile-99 of BMPR1a. The second pocket is formed by α-helix 1 of BMPR1a interacting with both BMP-2 subunits (Ile-62, Leu-66 and Val-70 from one and Val- 26, Trp-28, Trp31 and Tyr-103 from the other). In complex with the type 1 receptor, BMP remains a rigid ligand and does not undergo significant conformational change upon receptor binding (George P Allendorph et al. 2006).
BMP-2 and ActRII
The interface between BMP-2 and ActRII is a buried surface of 670A2. The interface involves generally hydrophobic residues, 12 from BMP-2 and 10 from the ActRII. Phe-42, Trp-60 and Phe-83 from ActRII form a hydrophobic core at the BMP-2/ActRII interface interacting with Ala-34, Pro-35, Ser-88, Met-89 and Leu-90 from BMP-2 (Gray et al. 2000). However, these residues complemented with other specific non-conserved residues outside this hydrophobic core may contribute to ligand specificity (George P Allendorph et al. 2006).
BMP crystal structures
Two co-crystal structures of BMP-BMP antagonist vividly demonstrate the similarities and differences in antagonist binding. The first co-crystal, BMP-7 in complex with Noggin, reveals a butterfly structure. The structure also reveals that the Noggin dimer forms a two-fold axis of symmetry with a head-to head conformation rather than the overlapping antiparallel conformation of its BMP ligand (Groppe et al. 2002). The Noggin clip extends and interacts with both wrist and knuckle residues, obstructing the BMP ligand to type I and type II receptor binding (Groppe et al. 2002).
The second co-crystal, BMP-2 in complex with von Willebrand type C (VWC1) domain of Crossveinless-2 (CV-2), shows considerable similarity in the prevention of Bone Morphogenetic Proteins (BMP) receptor binding, with CV-2 antagonist interactions occurring at both wrist and knuckle epitopes of BMP- 2. Sequence similarity in the clip regions of Noggin and CV-2, however, is not significantly shared (Zhang et al. 2008).
Follistatin
A third structure, Follistatin in complex with Activin, highlights further antagonistic diversity by blockade of type I and type II receptor binding sites by a peripheral clamp mechanism and not with clip domains as observed with Noggin and CV-2 (Lin et al. 2006; Thompson et al. 2005). The VWC1 domain of CV-2 is responsible for binding Bone Morphogenetic Proteins (BMPs) and is not only found in Chordin family members, but has also been identified in a diverse range of other extracellular proteins (Zhang et al. 2008). This X-ray resolved co-complex structure reveals the interaction of the VWC1 domain, but does not fully explain the intricacies of its binding. It still remains unclear as to how the linear peptide of the clip segment contributes strongly to the overall binding energy, yet is assumed to be highly flexible when unbound.
A second structural ensemble of VWC1 unbound to other proteins resolved by NMR revealed that the clip segment and a 30-residue subdomain termed SD1 of the VWC domain is preformed in its unbound state. The highly flexible nature of the clip segment exhibited strong affinity to BMP-2. The NMR structure showed that the N-terminal segment of the clip was flexible and disordered, whereas subdomain 1 exhibited a small and rigid three-stranded β sheet core. This rigidity contributed to the predefined orientation of the clip in a paperclip or hook-like architecture that brought the clip in close proximity to its final BMP binding site; therefore, likely lowering the overall binding energy cost and increasing affinity to the complex (Fiebig et al. 2013).
PRDC structure
The structure of protein related to DAN and Cerberus (PRDC), a potent BMP antagonist belonging to the DAN family, has also been resolved by X-ray crystallography. It shows that PRDC forms a non-covalent head-to tail growth factor-like dimer with an extensive hydrogen bond network between monomers (Nolan et al. 2013). Mutagenesis of PRDC identified residues belonging to the DAN domain on the convex surface, rather than the N-terminus, are critical for BMP binding affinity and that the N-terminus may only contribute weakly to ligand specificity. An N-terminal latch mechanism for BMP binding was therefore proposed due to the observed flexibility and potential for conformational sampling of the N-terminal domain that exposes the DAN domain residues upon interaction with a BMP ligand (Nolan et al. 2013).
NBL-1 Structure
The structure for the Bone Morphogenetic Protein (BMP)-antagonist (DAN family), Neuroblastoma Suppressor of Tumorigenicity 1 (NBL-1) was solved in 2015 (Nolan et al. 2015). It has long been known to antagonise BMP family members, specifically BMP-2, BMP-7 and GDF-7, with weaker potency than PRDC. The secondary and tertiary structure elucidation revealed an overall arch-like morphology similar to that of PRDC with two NBL-1 monomers forming an anti-parallel, non-covalent dimer (with an RMSD of 0.98A over the DAN domains when structurally aligned to PRDC).
Conserved with PRDC, the β2 sheet is integral for dimer formation. A network of 10 hydrogen bonds stabilises the overall structure. The main difference to PRDC is that the N-terminus of NBL-1 remains in a disordered state and does not interact with the DAN domain. In PRDC however, the N-terminus forms α helices which interact with the DAN domain by way of energetically favourable hydrophobic interactions (Nolan et al. 2015).
To investigate why NBL-1 was not as potent as PRDC, mutagenesis experiments were performed. The mutants were identified from multiple sequence alignments with PRDC. PRDC residues at positions critical for BMP antagonism were mutated into variants of NBL-1. Some of which were observed to increase Bone Morphogenetic Protein (BMP) antagonism compared to wild-type NBL-1. PRDC-like mutants were A58F and S67Y and were chosen as points for mutation due to being ‘strikingly non-conserved’ compared to PRDC. The elucidation of this structure revealed further insight to the structural relation to DAN family potency for BMP antagonism.
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024