Phosphatases and PTP1B – Mini review
Introduction
The human kinome is estimated to contain 518 genes, in comparison to the estimated 180 genes that comprise the phosphatome (Arena et al., 2005). These kinase genes represent a significant fraction of all eukaryotic genes, highlighting the prominent role of these enzymes in controlling key cellular functions (Manning et al., 2002). The first oncogene to be identified and characterised, Src, was found to be a tyrosine kinase (Collett et al., 1980; Czernilofsky et al., 1980). The isolation of this tyrosine kinase in 1980 came 7 years before the first tyrosine phosphatase, PTP1B, which was identified in 1987 and purified in 1988 (Lim Tung et al., 1987; Tonks et al., 1988). Investigations of the phosphatome, therefore, have lagged behind those of the kinome.
Phosphatases are enzymes which act antagonistically to kinases, by removing phosphate groups from their substrates. Phosphatases can act on various substrate types, for example lipid phosphatases such as PTEN (Maehama et al., 1998), and metabolic phosphatases including glucose-6-phosphatase (Cori et al., 2004) and alkaline phosphatase (Fishman et al., 1968). Protein phosphatases can be functionally divided into 2 superfamilies according to substrate preference; serine/threonine phosphatases (STPs), and the protein tyrosine phosphatases (PTPs). PTPs are a large, structurally diverse class of enzymes, all characterised by the presence of a 240 residue PTP catalytic domain containing a short signature motif (Tonks et al., 1996; Tiganis et al., 2007). The PTPs may be broadly divided into 2 subfamilies. Firstly, the dual-specificity phosphatases (DSP), which dephosphorylate not only on tyrosine residues but also on serine and threonines, and secondly, the tyrosine-specific phosphatase (TSP) subfamily.
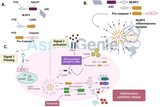
PTP1B is the archetypal member of the protein tyrosine phosphatase (PTP) superfamily, which possesses the catalytic domain and a short C-terminal targeting sequence (Anderie et al., 2007). The TSPs may be further subdivided into 2 categories based on function and subcellular localisation (Fig. 1.11). The clustering of sequences into divergent phylogenetic branches provides the basis for subdivision of the PTP family into 17 subtypes, the receptor subtypes R1-R8, and the non-transmembrane subtypes NT1-9 (Andersen et al., 2001). The receptor-like PTPs contain a transmembrane domain, and many possess distinct extracellular domains thought to facilitate the transduction of extracellular signals. The best-characterised member of the receptor-like PTPs is PTPRC, also known as CD45 or leukocyte common antigen (LCA), which is present on the outer membrane of leukocytes. The non-transmembrane PTPs are so named because they do not localise to the plasma membrane or possess extracellular domains, although some members of the family are in fact localised to the membranes of the ER and other subcellular organelles by transmembrane domains (Frangioni, 1992), and are thought to regulate a variety of intracellular events. PTPs act in opposition to intracellular protein tyrosine kinases (PTKs) to modulate the overall phosphorylation status of the proteome, though as of yet many PTPs are not well-characterised and their cognate substrates have not been determined (Tiganis et al., 2007). Much evidence has emerged in recent years to indicate that certain PTPs, particularly PTP1B and the closely-related TC-PTP, are regulators of malignant transformation and tumourigenesis (Stuible et al., 2008). Activating mutations in the related non-transmembrane PTP SHP-2 have been implicated in the causation of chronic myelogenous leukaemia (Tauchi et al., 1994; Tartaglia et al., 2004).
Phosphatase, Protein tyrosine phosphatase 1B (PTP1B)
PTP1B is the archetypal member of the PTP superfamily. It was the first human PTP to be characterised, and was identified independently by 2 sources. One group isolated an unknown 50 kDa phosphatase from bovine spleen which was characterised by its capacity to dephosphorylate an artificial substrate of poly(Glu, pTyr) (Lim Tung et al., 1987). At the same time, PTP1B was purified from human placenta, where it was named to differentiate 1A and 1B catalytic subunits isolated by affinity chromatography with a synthetic phospholysozyme substrate (Tonks et al., 1988). PTP1B was originally isolated from placental tissue which is a rich source of both insulin and the insulin receptor (Petruzzelli et al., 1982), a receptor tyrosine kinase (RTK) (Ullrich et al., 1985). It was hypothesised that an unknown PTP might be involved in the negative regulation of insulin receptor activity in placental tissue, as was found to be the case. As a consequence of its origins, much of the initial research into PTP1B focused on its interaction with the insulin receptor, and the functional consequences of their relationship in diabetes, related metabolic disorders, and obesity and satiety signalling (Koren et al., 2007; Oswal et al., 2009; Lantz et al., 2010).
PTP1B was demonstrated to be the major phosphatase of the insulin receptor, binding at numerous distinct phosphotyrosine sites (Seely et al., 1996; Salmeen et al., 2000). PTP1B-dependent dephosphorylation of the insulin receptor was found to antagonise insulin receptor signalling (Dadke et al., 2000; Dadke et al., 2001). In addition, reports published contemporaneously by several groups showed that the insulin receptor positively regulated PTP1B activity by tyrosine phosphorylation, indicating a negative regulatory feedback loop (Goldstein et al., 2000; Dadke et al., 2001). Conversely, PTP1B was also found to be negatively regulated by insulin-mediated, Akt-dependent phosphorylation at serine-50, suggesting a positive regulatory feedback loop (Ravichandran et al., 2001). These mechanistic insights were given clinical relevance by the creation of PTP1B-deficient transgenic mouse strains. PTP1B-deficient mice exhibit diminished diabetic pathology, have improved glycemic control, and are more resistant to diet-induced obesity than wild-type mice (Elchebly et al., 1999; Klaman et al., 2000). These discoveries led to a focus on PTP1B as an antagonist of insulin signalling in diabetes, and the development of PTP1B inhibitors as potential therapeutic strategies for the treatment of metabolic syndrome and diabetes (Koren et al., 2007; Lantz et al., 2010; Tiganis, 2012).
Phosphatase,PTP1B as a tumour suppressor and oncogene
The role of PTP1B as a tumour suppressor and cell death signalling component has garnered increasing attention in recent years. Initial reports suggested that PTP1B acted as a tumour suppressor, by antagonising the kinase activity of ErbB2/Her2/neu in breast and ovarian cancers (Wiener et al., 1994; Wiener et al., 1994b). ErbB2 is overexpressed in a third of breast cancers (Wiener et al., 1994), and transfection of ErbB2 into ovarian cancer cells resulted in the concomitant overexpression of PTP1B and several other PTPs (Wiener et al., 1996). Furthermore, PTP1B has been demonstrated to act in a pro-apoptotic manner during cell death induced by serum starvation of immortalised neonatal hepatocytes and brown adipocytes (Gonzalez-Rodriguez et al., 2007; Miranda et al., 2010). These two studies posited a role for PTP1B in the regulation of Bcl-2 protein expression; with PTP1B preventing the phosphorylation of Akt and subsequent phosphorylation of the transcriptional factor Foxo1, thus allowing the nuclear translocation of Foxo1 and the pro-apoptotic upregulation of Bim transcription. The close PTP1B homolog TC-PTP/PTPN2 was also found to upregulate Bim and promote apoptosis of pancreatic -cells in response to interferon stimulation (Santin et al., 2011), a further functional connection between PTP signalling and diabetes. The regulation of Akt by PTP1B was also implicated in the role of PTP1B in death due to hypoxia/reoxygenation stress of rat cardiomyocytes, although a specific role for Bim was not elucidated in this study (Song et al., 2008).
In contrast to its role as an antagonist of oncogenic kinase signalling, PTP1B has also itself been shown to regulate numerous pathways in an oncogenic manner. PTP1B is overexpressed in over 90% of breast cancers (Wiener et al., 1994), and the inhibition of PTP1B has been found to dramatically delay or prevent the initiation of Her2/neu-induced neoplasia in mice (Bentires-Alj et al., 2007; Julien et al., 2007; Stuible et al., 2008). The growth and metastasis of breast cancer is similarly regulated by PTP1B, which regulates c-Src in the growth of invadopodia, membrane protrusions which facilitate metastasis in solid tumour tissues (Cortesio et al., 2008). The PTP1B regulation of Src is a potentially highly therapeutically-relevant aspect of its oncogenic signalling. PTP1B is the primary phosphatase of Src in several breast cancer cell lines, dephosphorylating c-Src at tyrosine-529 (Bjorge et al., 2000), and removing the inhibitory effect of this phosphorylation. This PTP1B-dependent activation of Src has been demonstrated to be a requirement for integrin signalling in both platelets (Arias-Salgado et al., 2005), and fibroblasts by the formation of a complex with the adaptor protein p130Cas (Liang et al., 2005). Overexpression of PTP1B was found to be causative for the upregulation of Src in colon cancer cell lines, and this upregulation and activation of Src was proposed to increase tumourigenicity in colon cancer neoplasia (Zhu et al., 2007). In mitochondria isolated from numerous different rat tissue types, PTP1B, the related PTP SHP-2, and Src were found only to be localised to the mitochondria in neurons (Arachiche et al., 2008), and it was subsequently found that PTP1B dephosphorylates Src at the mitochondria (HǸbert Chatelain et al., 2011), where it is thought to modulate oxidative phosphorylation/ATP production, indicating the significance of this regulation in multiple subcellular organelles. It has previously been demonstrated that Src itself can phosphorylate PTP1B in vitro, suggesting the possibility of a feedback regulatory loop (Jung et al., 1998).
In addition to modulating Src activity, PTP1B is implicated in the oncogenic promotion of Ras signalling via the p62Dok protein. p62Dok is a negative regulator of both Erk and Ras signalling, as well as a substrate of PTP1B, and is hyperphosphorylated in PTP1B-deficient cells (DubǸ et al., 2004). Enhanced phosphorylation of p62Dok in PTP1B tumours correlated with decreased phosphorylation and increased levels of p120RasGAP protein (Julien et al., 2007), ultimately resulting in increased activity of the Ras signalling pathway. It is thought that this activation synergises with the effect of PTP1B on ErbB2/Her2/neu mentioned previously, as ErbB2 is itself a positive regulator of Ras (Tonks et al., 2007). In addition to the well-characterised regulation of Src and Ras, PTP1B mediates its activity through a range of receptor tyrosine kinases (RTKs), including PDGF-R (Haj et al., 2002; Mertins et al., 2008), EGF-R (Haj et al., 2002), and IGF-R (Kenner et al., 1996; Buckley et al., 2002) (Hoekstra et al., 2012), and particularly the insulin receptor, where the majority of research into PTP1B function has been focused, as previously discussed (Tiganis, 2012). Indeed, a number of PTP1B inhibitors are currently in preclinical trials for the treatment of diabetes, obesity and related metabolic disorders (Lantz et al., 2010; Verspohl, 2012). It is thought that PTP1B inhibitors which are currently at an advanced stage of development could be repurposed for the treatment of certain cancers (Tonks et al., 2007).
The pharmacological relevance of this inhibition as an adjuvant therapy has already been demonstrated by the synergistic activity of the PTP1B inhibitor DMHV (or bis[N,N-Dimethylhydroxamido]hydroxooxovanadate) and the Bcr-Abl inhibitor Glivec (STI-571) in the induction of cell death in K562 chronic myelogenous leukemia (CML) cells (Koyama et al., 2006). Conflicting reports indicate that PTP1B inhibition can result in either increased Bcr-Abl activity and chemotherapeutic insensitivity (LaMontagne et al., 1998; Koyama et al., 2006), or the ubiquitination and degradation of Bcr-Abl (Alvira et al., 2011). Recently, it has been demonstrated that PTP1B transcription is upregulated by treatment of prostate cancer cell lines with androgen, suggesting a potential role for PTP1B inhibitors in the treatment of prostate cancer (Lessard et al., 2012). Clearly, it will be necessary to elucidate the precise cellular roles in which PTP1B is acting in different tissue and cancer types, in order to develop efficacious and appropriate therapeutic responses. PTP1B, as previously discussed, is implicated in a range of both metabolic and cell survival/cell death signalling pathways. In addition to these pathways, PTP1B regulates the activity of many receptor tyrosine kinases, including the PDGF receptor and EGF receptor, as well as the IGF receptor, and of course the insulin receptor where the preponderance of literature relating to PTP1B is focused (Salmeen et al., 2000; Buckley et al., 2002; Mertins et al., 2008). Taken collectively, literature reports have demonstrated that PTP1B can influence many separate signalling pathways, which has led to its investigation as both a tumour suppressor, and an oncogene itself. Although it is unlikely that all PTP1B substrates have been identified, use of substrate-trapping PTP1B mutants in conjunction with quantitative mass spectrometry have elucidated a substantial number of pathways which are regulated by PTP1B(Lessard et al., 2010; LabbǸ et al., 2012).

window.SHOGUN_IMAGE_ELEMENTS = window.SHOGUN_IMAGE_ELEMENTS || new Array(); window.SHOGUN_IMAGE_ELEMENTS.push({ hoverImage: '', uuid: 's-442ef777-e191-442a-9d14-fde6d5952af2' })
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024