Chronic myelogenous leukaemia (CML) – Review
Chronic myelogenous leukaemia (CML) is a myeloproliferative haematopoietic malignancy, characterised by a karyotypic abnormality present in more than 95% of cases known as the Philadelphia chromosome (Lozzio et al., 1975). CML has its origins in a common myeloid progenitor cell, which differentiates into erythrocytes, megakaryoctes, or granulocytes/monocytes (Fialkow et al., 1977).
The Philadelphia Chromosome
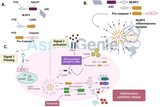
The Philadelphia chromosome was first karyotypically characterised in 1959 (Moorhead et al., 1960). It was demonstrated to be the result of a reciprocal translocation between the long arms of chromosomes 9 and 22 (Rowley, 1982).This translocation results in the transfer of the Abelson (abl) proto-oncogene on chromosome 9 to an area of chromosome 22 referred to as the breakpoint cluster region (BCR) (Bartram et al., 1983). This chromosomal rearrangement results in increased transcription of an oncogenic fusion RNA known as bcr-abl (Shtivelman et al., 1985), which encodes Bcr-Abl, a constitutively-activated cytosolic tyrosine kinase (Fainstein et al., 1987).
During the 1970s and 1980s virally encoded oncogenes were shown to have cellular equivalents in humans that became altered in human cancers. The molecular characterisation of the Philadelphia chromosome was aided by the observation that the Abelsonmurine leukaemia retrovirus (v-Abl) human homologue, c-Abl, localised to the long arm of chromosome 9 [Heisterkamp et al., 1982].Using c-Abl and v-Abl hybridization probes it was observed that the Abl probes bound to chromosome 22 (on the Philadelphia chromosome) instead of their normal position on chromosome 9, while regions of the c-sis oncogene, which is normally found on chromosome 22 had translocated to chromosome 9 in CML cells [de Klein et al., 1982; Groffen et al., 1983].
Together these studies revealed that a reciprocal translocation was responsible for the Philadelphia chromosome and suggested the possible transforming potential of the c-Abl proto-oncogene [Pane et al., 2002]. Cloning of the Bcr/Abl breakpoint was a major breakthrough in the characterisation of CML. Further studies revealed that Abl translocated to a major break cluster region (M-bcr) on chromosome 22 and resulted in a fusion gene between Bcr and cAbl [Groffen et al., 1984]. The finding that the Bcr-Abl fusion mRNA encoded a 210 kDa constitutively active tyrosine kinase, that could transform cells both in vitro and in vivo confirmed that CML was a result of the Bcr-Abl fusion protein [Pane et al., 2002].Demonstrating that viral heterologous expression of the Bcr-Abl construct was necessary and sufficient for the emergence of a CML phenotype in a murine model (Hariharan et al., 1989). Which confirmed the central role of Bcr-Abl as the key oncogenic signalling event driving CML pathogenesis.
Clinical Phases of CML
CML generally progresses through three distinct clinical phases: A chronic, accelerated and blast crisis phase.
- Chronic phase (CP) – Chronic phase occurs in patients which are often only moderately symptomatic. During the chronic phase p53 activity is maintained and mature granulocytes are still produced. However, an increased number of myeloid progenitor cells are present within the blood. As the disease progresses patients enter an accelerated phase (AP).
- Accelerated phase (AP) – The acclerated phase is characterised by splenomegaly, abnormal platelet production, and increased production of myeloblast cells leading to blast crisis.
- Blast Crisis (BC) – Blast crisis is characterised by greater than 20% of myeloblasts or lymphoblasts in the bone marrow, typically forming large clusters (Faderl et al., 1999), and p53 is frequently inactivated by mutation or loss of expression (Ahuja et al., 1989).During BC haematopoietic differentiation is arrested and immature blasts accumulate in the bone marrow and enter the blood circulation with blast crisis cells also exhibiting karyotype alterations and genomic instability [Melo and Barnes, 2007].
The three phases of CML can be modelled within the laboratory setting using cell lines derived from patients in the chronic, accelerated and blast crisis phases of CML.
Bcr-Abl Signalling
The Bcr-Abl fusion protein exists as a 210 kDa protein in CML and a 185 kDa protein in ALL. The constitutive activation of Bcr-Abl results in the transformation of the cell through the activation of cell survival and cell death resistance pathways. Bcr-Abl interacts with many signalling proteins through various functional domains and motifs and leads to the phosphorylation of paxillin, phosphoinositide 3-kinase (PI3K), RAS GTPase activating protein, focal adhesion kinase and the signal transducer and activator of transcription 5 (STAT5) [Ren, 2005]. These proteins subsequently activate a diverse range of signalling pathways which activate proteins such as JNK (c-Jun N-terminal kinase), PI3K, RAS and the RAC-alpha Ser/Thr-protein kinases (AKT), as well as transcription factors including STAT5, Myc and nuclear-factor kB. Furthermore, Bcr-Abl signalling upregulates the anti-apoptotic protein, Bcl-XL, therefore increasing the survival potential of the cell [Ren, 2005].
Bcr-Abl signalling has also been proposed to induce mutations resulting in genomic instability which may account for the occurrence of non-random chromosomal abnormalities associated with advanced CML progression, such as trisomy 8, trisomy 21, additional Ph chromosome, loss of the Y chromosome, monosomy 7 and isochromosome 17 [Melo and Barnes, 2007].
STI571 and Bcr-Abl resistance
Treatment of patients with CML over the past 60 years has involved a range of therapeutic approaches. In the past CML patients had an average of 3-years life expectancy, with less than 20% of patients surviving 5 years after diagnosis. Many approaches to CML treatment have been taken including radiotherapy, treatment of patients with the DNA synthesis inhibitor hydroxyurea and interferon-y treatment. However, only allogenic bone marrow transplantation in the chronic stage is the curative therapy for CML [Faderl et al., 1999].
STI571- a tyrosine kinase inhibitor
In 1996 a landmark approach to cancer therapy was made by Druker and colleagues, through the discovery of a highly specific tyrosine kinase inhibitor of Bcr-Abl called STI571 (Imatinib mesylate/Gleevec). STI571 was found to inhibit the ATP-binding site of the Abl kinase, thus inhibiting the constitutive tyrosine kinase activity of Bcr-Abl [Druker et al., 1996]. STI571 was the first known therapeutic to directly inhibit the activity of an oncogene and represented a major breakthrough in new approaches to cancer treatment. Phase I clinical trials with STI571 commenced in 1998, which lead to its approval as curative measure for CML in 2001 by the Food and Drug Administration (FDA) [Druker et al., 2001]. A complete cytogenetic response (CCR) was detected in up to 40% of patients in chronic phase CML who had undergone interferon-y treatment. Furthermore up to 80% of newly diagnosed patients displayed CCR; however, blast crisis patients presenting with CCR and residual disease often relapse even with continued treatment [Deininger et al., 2005].
STI571 Resistance
Advanced stages of CML are commonly associated with the emergence of Bcr- Abl mutated clones which are refractory to STI571 treatment. Up to 60 Bcr-Abl mutations that confer various degrees of resistance to STI571 treatment have been identified. The gatekeeper T315I mutation enables STI571 resistance through the loss of a critical hydrogen bond contained within a threonine residue that is required for STI571 binding and the addition of a hydrocarbon group on isoleucine which results in steric hindrance, and thus the inhibition of STI571 binding [Gorre et al., 2001]. Recently microtubule targeting agents have shown promising results in the treatment of ex vivo CML patient samples that are resistant to STI571 and therefore may represent a promising therapy for CML treatment [Bright et al., 2010].
Glivec as a paradigm of CML treatment
The development of the Bcr-Abl inhibitor Glivec by Brian Druker and Ciba-Geigy (now Novartis) as a therapeutic strategy for the treatment of CML (Druker et al., 1996) was a milestone in the rational design of anti-cancer drugs which specifically target an oncogenic signalling pathway (Druker, 2002). Glivec exhibits a high degree of efficacy in CML patients during chronic and acute phases of the disease where up to 80% of these patients exhibit a complete cytogenetic response (Druker et al., 2009). However, therapeutic efficacy is reduced in blast crisis, and many patients who exhibit a cytogenetic response undergo relapse due to the persistence of leukemic stem cells.
Glivec Resistance
Furthermore, Glivec resistance can occur via several mechanisms; the upregulation of Bcr-Abl levels (Weisberg et al., 2000); specific point mutations of Bcr-Abl at sites that abrogate Glivec binding (von Bubnoff et al., 2002); downregulation of the OCT cation transporter which mediates active transport of Glivec into the cancer cell (Hiwase et al., 2008); or by upregulation of the P-glycoprotein efflux pump which is responsible for multi-drug resistance (Illmer et al., 2004). These drawbacks in the therapeutic efficacy have led to both the development of novel compounds based on the chemical structure of Glivec, including the therapeutically-promising compounds nilotinib and the dasatinib (Weisberg et al., 2007), and the evaluation of existing chemotherapeutics as potential adjuvant therapies.
Glivec-adjuvant therapy
Diverse pathways have been investigated for potential efficacy as a Glivec-adjuvant therapy for CML, including inhibition of MAP kinase signalling (Yu et al., 2002), and Src signalling (Gu et al., 2005), or the inhibition of autophagy (Mishima et al., 2008). However, the most promising avenue of adjuvant therapy has emerged from studies investigating the synergistic cell death induced by the co-administration of Glivec and an adjuvant MTA. For example, Epothilone B was found to potentiate Glivec anti-tumour activity in mice and rats without significant increase in morbidity (Pietras et al., 2003; OReilly et al., 2005). Furthermore, the novel pyrrolo-1,5-benzoxazepine (PBOX) compounds PBOX-6 (Greene et al., 2007), and PBOX-21 (Bright et al., 2009), were both found to potentiate Glivec-mediated programmed cell death in the K562 CML cell line.
A similar effect was reported for the Glivec/docetaxel treatment of K562 cells (Gucluler et al., 2009). The success of these studies in producing a synergistic anti-neoplastic effect, by combining Glivec and various MTAs, highlights the therapeutic efficacy of simultaneously targeting different facets of the transformed cell. In these examples, the cell death effects of MTA treatment are potentiated by the inhibition of the Bcr-Abl oncogenic pathway. Increased understanding of the precise molecular mechanisms underpinning MTA activity will hopefully facilitate the design of new targeted anti-cancer drugs.
The K562 CML cell model
K562 is a transformed cell line, first cultured in the 1970s from a 53 year old female CML patient in blast crisis (Lozzio et al., 1975). Unsurprisingly given its origin from a transformed myeloid progenitor cell, the K562 cell line can spontaneously develop characteristics similar to early-stage erythrocytes or granulocytes/monocytes, and erythroid differentiation can be induced by the treatment of K562 with high concentrations of dimethyl sulfoxide (DMSO) or sodium butyrate (Andersson et al., 1979; Andersson et al., 1979). In keeping with its representation of CML blast crisis, K562 expresses a non-functional frame-shift mutant of p53 (Law et al., 1993). It is thought that the loss of p53 function is a secondary consequence of Bcr-Abl oncogenic signalling, which serves to increase the invasiveness and proliferative potential of CML cells as the disease progresses.
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024