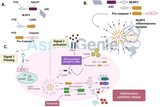
Antigen Presenting Cells (APCs) and cancer immunotherapy
Explore antigen presentation's crucial role in adaptive immunity and its impact on cancer immunotherapy, highlighting the diversity and function of MHC class I molecules.
Key Takeaways
- Antigen presentation by MHC class I is key for adaptive immunity.
- Specialized cells, antigen-presenting cells (APCs), present antigens to T cells.
- MHC-I molecules' polymorphism enables diverse antigen presentation.
- Cancer immunotherapy leverages antigen presentation to target tumor cells.
What is Antigen Presentation?
Our immune system has various mechanisms to defend against pathogens, with adaptive immunity being a crucial component. Adaptive immunity enables our immune system to mount specialized and targeted immune responses against specific pathogens. One key pathway involved in this process is the MHC class I antigen presentation pathway, as discovered by Bjorkman et al. in 1987a,b. While it may sound complex, the underlying concept is quite straightforward. Proteins within our cells undergo constant degradation to facilitate recycling. Interestingly, a portion of these degradation products consists of shorter peptides, typically composed of 8 to 11 amino acids. Rather than being recycled, these peptides serve a different purpose—they are utilized by the immune system itself, as noted by Michalek et al. in 1993. Specifically, they bind to a receptor known as MHC class I molecule (MHC-I), subsequently migrate to the cell surface, and become presented by specialized immune cells called antigen-presenting cells (APCs). This presentation allows the immune system to recognize and respond to potential threats effectively.
The process of MHC class I antigen presentation is vital for eliciting a potent immune response against pathogens. After the degraded peptides are bound to MHC-I molecules, they undergo careful quality control checks within the endoplasmic reticulum (ER) of the cell. This ensures that only properly formed peptide-MHC complexes are transported to the cell surface for display. This rigorous selection process guarantees that only peptides derived from intracellular proteins, such as those produced by viral or tumor antigens, are presented to the immune system. By presenting a diverse repertoire of peptides, APCs play a crucial role in stimulating Cytotoxic T cells are a type of T cell that is responsible for killing infected or cancerous cells. They are distinguished by the expression of the activation marker CD8. (CTLs) to recognize and eliminate infected or malignant cells. This process of antigen presentation by APCs acts as a crucial bridge between the innate and adaptive immune responses, ultimately bolstering the body's defenses against pathogens and cancer cells.
What are Antigen Presenting Cells?
Almost all nucleated cells display MHC-I molecules at the cell surface, there are however cells of the immune system that are specialized on antigen presentation, they are professional antigen presenting cells (Shastri & Yewdell 2015). Other cells of the immune system, like T cells or NK cells, survey those antigen presenting cells. If the displayed peptide is derived from a naturally occurring protein the immune system senses the surveyed cell is intact and functioning. However, if the peptide is derived from a virus, the T cell will sense that as a danger signal, and will proceed to kill the virus infected cell (Shastri et al. 2002). Obviously, I painted a very simplified picture, so I will explain a few of the key components and stages before talking about cancer immunotherapy: MHC-I molecules, peptide generation, loading and transport of the complex.
The expression of MHC-I molecules on almost all nucleated cells enables the immune system to constantly survey the body's cells for any abnormalities or signs of infection. This constant surveillance is crucial in maintaining immune homeostasis and swiftly responding to any threats. Professional APCs, such as dendritic cells, macrophages, and B cells, possess unique adaptations that allow them to efficiently capture, process, and present antigens to T cells. These APCs excel at internalizing pathogens or foreign substances, breaking them down into smaller fragments, and loading the resulting peptide fragments onto MHC-I molecules within specialized compartments called endosomes or phagosomes. This process, known as antigen processing and presentation, enables APCs to present a diverse repertoire of antigens to T cells, effectively engaging the adaptive immune response.
Furthermore, the presentation of viral peptides on MHC-I molecules serves as a critical mechanism for immune surveillance. When a virus infects a host cell, it hijacks the cellular machinery to produce viral proteins. These viral proteins can be degraded into peptides and loaded onto MHC-I molecules for presentation at the cell surface. T cells, particularly CD8+ cytotoxic T lymphocytes (CTLs), possess receptors called T cell receptors (TCRs) that specifically recognize and bind to viral peptide-MHC-I complexes. This interaction triggers an immune response, leading to the elimination of virus-infected cells by CTLs.
MHC-I Molecules
MHC-I molecules are peptide receptors that can bind peptides 8−11 amino acids long (Bjorkman et al. 1987b). What is special about them, is that they are the most polymorphic genes in the human genome, there are over 5000 individual variants and they vary immensely between individuals and populations (Klein 1986, Kulski et al. 2002, Kelley et al. 2005). Every person has between 3 and 6 different variants and they are different mainly in the part of the molecule that binds peptides. This means that every MHC-I molecule is able to bind its own set of peptides generating a unique complex. This way our total pool of MHC-I molecules protects against a variety of diseases.
The extensive polymorphism of MHC-I molecules is crucial for the immune system's ability to respond effectively to a wide range of pathogens. The diverse array of MHC-I variants within a population ensures that different individuals can present a broad spectrum of pathogenic peptides to their immune cells. This genetic diversity acts as a safeguard against pathogens' attempts to evade immune recognition by rapidly mutating their antigenic targets. The extraordinary variability of MHC-I molecules increases the likelihood that at least a subset of the population will possess the necessary MHC-I variants to mount an immune response against a given pathogen or tumor antigen.
Furthermore, the unique set of peptides presented by each individual's MHC-I molecules plays a crucial role in shaping the immune response. By presenting a diverse repertoire of antigens, MHC-I molecules contribute to the generation of a robust and adaptable immune system. This peptide presentation diversity is particularly important in the context of cancer immunotherapy, where efforts are made to harness the immune system's ability to recognize and eliminate cancer cells.
MHC Class - I
Peptide Generation
Where do the peptides that bind to MHC-I molecules come from and how are they generated? Proteins in the cytoplasm are broken down into smaller fragments by the proteasome, a big protein degradation complex (Gromm e & Neefjes 2002). Those peptides are then transported into the endoplasmic reticulum by the TAP transporter, a kind of molecular pump, where they can be loaded onto MHC-I molecules (Gromme & Neefjes 2002). In the ER there are different enzymes that can further trim the transported peptides prior to loading and therefore further contribute to the diversity of peptides generated (Nagarajan et al. 2016).
The trimming of peptides within the ER is carried out by various peptidases, including ERAP1 (ER aminopeptidase 1) and ERAP2. These enzymes selectively remove amino acids from the N-terminus of the peptides, further shaping the peptide pool available for MHC-I binding. The trimming process is not random but is influenced by the specific preferences of these peptidases, such as their substrate specificity and cleavage preferences. Consequently, the final repertoire of peptides displayed by MHC-I molecules on the cell surface is a result of a complex interplay between proteasomal degradation, TAP-mediated peptide transport, and ER-resident peptidases.
It is worth noting that the diversity of peptides generated through this process is crucial for the immune system's ability to recognize a broad range of pathogenic and tumor antigens. The variations in peptide length and sequence contribute to the broad specificity of MHC-I molecules, allowing them to present a vast array of antigens to immune cells. This diversity is instrumental in eliciting robust immune responses and enabling the immune system to identify and eliminate infected or malignant cells more effectively.
MHC-I Loading and Transport
Antigen presenting cells go to great lengths to ensure that MHC-I molecules are loaded with the correct peptide. After loading MHC-I molecules undergo rigorous quality control, so that only stable complexes end up at the cell surface. The first step in this process is the peptide loading complex (PLC). It is a multi protein complex that stabilizes MHC-I molecules and facilitates loading. One of the molecules within the PLC, tapasin, competes with bound peptides, and has the ability to dissociate low-affinity peptides from MHC-I molecules. Only when a medium/high-affinity peptide is bound the complex will leave the PLC (Blees et al. 2015). Following the secretory pathway from the ER through the Golgi to the cell surface the peptide-MHC complex encounters one additional quality control checkpoint. A protein called TAPBPR, that is fairly similar to tapasin, also competes with the bound peptide (Hermann et al. 2015). If the peptide that is bound to MHC-I does not have a high enough affinity, it will dissociate and the empty MHC-I molecule will be sent back to the PLC to be loaded with a new peptide. MHC-I molecules can be found on almost every nucleated cell in the body, including cancer cells. This makes them an excellent target for therapies (Snyder et al. 2014).
Antigen Presentation and Cancer (Immunotherapy)
So how does this pathway link to cancer immunotherapy? It was long be- lieved that the immune system tells apart ”self” from ”non-self” because it is trained early in life not to attack the cells of your own body . Since cancer cells come from your own body, you would assume that the immune system cannot detect cancer. However, this idea was amended as there are numerous examples of the immune system recognizing ”self”. The current notion is that the immune system is there to differentiate between harmless and potentially harmful signals, which can include either proteins that are expressed in much higher abundance than they normally would, or proteins that bear mutations for example due to cancer (Matzinger 1994, Granados et al. 2015). It was also shown that the immune system is able to start an immune response towards cer- tain cancers, however, it is not powerful enough to fight the cancer completely (Banchereau & Palucka 2005). This is due to a number of reasons, including the high mutation rate of cancers. Peptides that are generated from proteins that bear mutations are called ”tumor neoantigens”.
Tumour Neoantigens
There are currently hundreds of research groups and companies racing to identify tumor neoantigens and to build algorithms that accurately predict which peptides will be generated and bound to MHC-I (Editorial 2017, Wang & Wang 2016, Schumacher & Schreiber 2015). However, there are numerous challenges in this endeavour: First, MHC-I molecules are highly diverse and sufficient data are lacking for many of them. Another issue is that many predicted peptides cannot be identified in tumor samples, or they fail to elicit T cell responses. This might be due to different reasons, one of which is that the rules of peptide selection are still poorly under- stood. For example, only recently was a new quality control checkpoint in the MHC-I antigen presentation pathway identified that substantially influences the peptide repertoire (Neerincx et al. 2017). Therefore, there needs to be a much deeper understanding of peptide selection and the antigen presentation path- way to make cancer immunotherapy as effective as it can be. Tumor neoantigen discovery, and systematic understanding of the rules of peptide selection, will lead to personalized cancer immunotherapies that boost the patient’s immune system and help them fight cancer.
In conclusion, antigen-presenting cells (APCs) play a crucial role in cancer immunotherapy by presenting tumor antigens to the immune system and initiating targeted immune responses against cancer cells. Through the MHC class I antigen presentation pathway, APCs display degraded peptides derived from intracellular proteins on their cell surface, enabling the immune system to recognize and eliminate cancerous cells. The identification of tumor neoantigens and the understanding of peptide selection rules are ongoing areas of research that hold immense promise for personalized cancer immunotherapies. By harnessing the power of APCs and enhancing our knowledge of the intricate mechanisms involved, we can strive to develop more effective and tailored immunotherapeutic approaches, bolstering the patient's immune system and unleashing its potential to fight against cancer. APCs and cancer immunotherapy represent a rapidly advancing field that continues to offer new avenues for combating this complex disease.
References
Banchereau, J. & Palucka, a. K. (2005), ‘Dendritic cells as therapeutic vaccines against cancer.’, Nature reviews. Immunology 5(4), 296–306.
Bjorkman, P. J., Saper, M. A., Samraoui, B., Bennett, W. S., Strominger, J. L. & Wiley, D. C. (1987a), ‘Structure of the human class I histocompatibility antigen, HLA-A2’, Nature 329(6139), 506–512.
Bjorkman, P. J., Saper, M. A., Samraoui, B., Bennett, W. S., Strominger, J. L. & Wiley, D. C. (1987b), ‘The foreign antigen binding site and T cell recogni- tion regions of class I histocompatibility antigens.’, Nature 329(6139), 512–8.
Blees, A., Reichel, K., Trowitzsch, S., Fisette, O., Bock, C., Abele, R., Hummer, G., Sch ̈afer, L. V. & Tamp ́e, R. (2015), ‘Assembly of the MHC I peptide- loading complex determined by a conserved ionic lock-switch’, Scientific Re- ports 5, 17341.
Editorial (2017), ‘The problem with neoantigen prediction’, Nature Biotechnol- ogy 35(2), 97–97.
Granados, D. P., Laumont, C. M., Thibault, P. & Perreault, C. (2015), ‘The nature of self for T cells-a systems-level perspective’, Current Opinion in Immunology 34, 1–8.
Gromm ́e, M. & Neefjes, J. (2002), ‘Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways’.
Hermann, C., Trowsdale, J. & Boyle, L. H. (2015), ‘TAPBPR: A new player in the MHC class I presentation pathway’, Tissue Antigens 85(3), 155–166.
Kelley, J., Walter, L. & Trowsdale, J. (2005), ‘Comparative genomics of major histocompatibility complexes’.
Klein, J. (1986), ‘Antigen-major histocompatibility complex-T cell receptors: inquiries into the immunological m ́enage `a trois.’, Immunologic research 5(3), 173–90.
Kulski, J. K., Shiina, T., Anzai, T., Kohara, S. & Inoko, H. (2002), ‘Com- parative genomic analysis of the MHC: the evolution of class I duplication blocks, diversity and complexity from shark to man.’, Immunological reviews 190(1), 95–122.
Matzinger, P. (1994), ‘TOLERANCE, DANGER, AND THE EXTENDED FAMILY’, Annu. Rev.lmmunol 12, 991–1045.
Michalek, M. T., Grant, E. P., Gramm, C., Goldberg, A. L. & Rock, K. L. (1993), ‘A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation’, Nature 363(6429), 552–554.
Nagarajan, N. A., de Verteuil, D. A., Sriranganadane, D., Yahyaoui, W., Thibault, P., Perreault, C. & Shastri, N. (2016), ‘ERAAP Shapes the Pep- tidome Associated with Classical and Nonclassical MHC Class I Molecules.’, Journal of immunology (Baltimore, Md. : 1950) .
Neerincx, A., Hermann, C., Antrobus, R., van Hateren, A., Cao, H., Trautwein, N., Stevanovi?, S., Elliott, T., Deane, J. E. & Boyle, L. H. (2017), ‘TAPBPR bridges UDP-glucose:glycoprotein glucosyltransferase 1 onto MHC class I to provide quality control in the antigen presentation pathway’, eLife 6, e23049.
Schumacher, T. N. & Schreiber, R. D. (2015), ‘Neoantigens in cancer im- munotherapy’, Science 348(6230), 69–74.
Shastri, N., Schwab, S. & Serwold, T. (2002), ‘Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules’, Annu Rev Immunol 20(1), 463–493.
Shastri, N. & Yewdell, J. W. (2015), ‘Editorial overview: Antigen processing and presentation: Where cellular immunity begins’, Current Opinion in Im- munology 34, v–vii.
Snyder, A., Makarov, V., Merghoub, T., Yuan, J., Zaretsky, J. M., Desrichard, A., Walsh, L. A., Postow, M. A., Wong, P., Ho, T. S., Hollmann, T. J., Bruggeman, C., Kannan, K., Li, Y., Elipenahli, C., Liu, C., Harbison, C. T., Wang, L., Ribas, A., Wolchok, J. D. & Chan, T. A. (2014), ‘Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma.’, The New England journal of medicine 371(23), 2189–2199.
Wang, R.-F. & Wang, H. Y. (2016), ‘Immune targets and neoantigens for cancer immunotherapy and precision medicine’, Nature Publishing Group 27(1), 11– 37.
Written by Pragna Krishnapur
Pragna Krishnapur completed her bachelor degree in Biotechnology Engineering in Visvesvaraya Technological University before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024