Immunoglobulin vs Antibody: Unveiling the Intricacies
In the realm of immunology, the terms "immunoglobulin" and "antibody" often find themselves used interchangeably, sparking confusion among those keen on understanding the immune system's fine details. While closely related, these terms encapsulate nuances vital for a comprehensive grasp of how the body defends itself against pathogens. This article embarks on a deep dive into the definitions, functions, structures, and types of immunoglobulins and antibodies, unveiling their similarities and differences.
Defining the Players
Immunoglobulin (Ig)
Immunoglobulins, commonly abbreviated as Igs, are glycoprotein molecules produced by plasma cells (a type of white blood cell). They play a pivotal role in detecting and binding to antigens (foreign substances that trigger an immune response), such as bacteria and viruses. Immunoglobulins are the physical manifestation of antibodies, serving as the basis for the immune system's ability to recognize and remember pathogens.
Antibody
An antibody refers to the immune system's response to an antigen. In essence, antibodies are a subset of immunoglobulins that are specifically produced in response to and counteract a specific antigen. They are the "soldiers" that seek out and mark pathogens for destruction by other immune cells.
Immunoglobulin and Antibody: Unraveling the Relationship
While every antibody is an immunoglobulin, not every immunoglobulin serves as an antibody. This distinction hinges on the function and specificity of the immunoglobulin in the immune response. Immunoglobulins include both the antibodies that are actively involved in the immune response and those that serve other roles within the immune system, such as cell signaling.
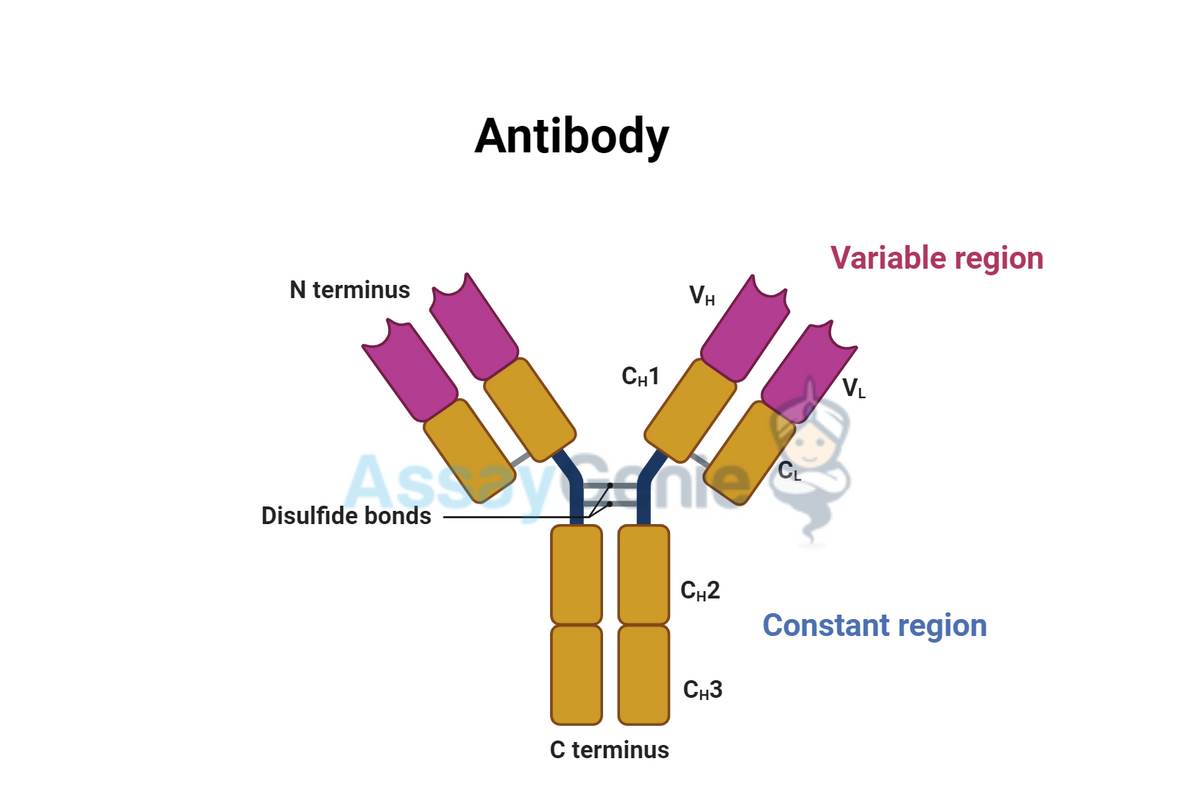
Structure and Function
Both immunoglobulins and antibodies share a common structure, composed of four peptide chains: two heavy chains and two light chains, connected by disulfide bonds. This structure forms a Y-shape, which is crucial for their ability to bind to antigens. However, the specific sequences within these chains can vary immensely, allowing for the incredible diversity in antigen recognition.
The Types of Immunoglobulins
Immunoglobulins are categorized into five primary classes, each serving unique roles in the immune system:
IgG: The most common type, responsible for the majority of antibody-based immunity against pathogens.
IgA: Found in mucous membranes, guarding the entrances of the body.
IgM: The first type produced in response to an infection.
IgE: Involved in allergic reactions and defense against parasitic infections.
IgD: Functions mainly as an antigen receptor on B cells that have not been exposed to antigens.
Immunoglobulin and Antibody: A Comparative Table
| Feature | Immunoglobulin (Ig) | Antibody |
| Definition | Glycoproteins produced by plasma cells that can bind to antigens. | A type of immunoglobulin that specifically binds to and neutralizes antigens. |
| Role | Includes a broader category of molecules involved in antigen recognition and immune response. | Primarily involved in identifying and neutralizing specific pathogens. |
| Types | Five main classes (IgG, IgA, IgM, IgE, IgD) based on function and location. | All antibodies are immunoglobulins, but classified based on their antigen specificity rather than structure. |
| Function | Not all are directly involved in the attack on pathogens; some have regulatory or signaling roles. | Directly involved in the immune defense against pathogens by marking or neutralizing them. |
Conclusion
The intricacies of immunoglobulins and antibodies reveal the complexity of the immune system's arsenal against pathogens. Understanding the distinctions and relationships between these components not only enriches our knowledge of immunology but also underscores the precision of our body's defense mechanisms. As research progresses, the depth of our comprehension of these molecules continues to expand, promising advancements in medical science and treatments.
References
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Section 24.2, Antibodies Can Be Degraded into Fragments That Retain Antigen-Binding Activity.
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Chapter 4, Immunoglobulin Structure and Function.
- Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 9th edition. Philadelphia: Elsevier; 2018. Chapter 5, Immunoglobulin Structure and Function.
- Roitt I, Brostoff J, Male D. Immunology. 6th edition. Philadelphia: Mosby; 2001. Chapter 3, The Production of Antibodies.
- Murphy K, Weaver C. Janeway's Immunobiology. 9th edition. New York: Garland Science; 2016. Chapter 4, Antibody Structure and the Generation of B-Cell Diversity.
- Parham P. The Immune System. 4th edition. New York: Garland Science; 2014. Chapter 5, Immunoglobulin Genes.
- Male D, Brostoff J, Roth DB, et al., editors. Immunology. 8th edition. Philadelphia: Elsevier Saunders; 2012. Chapter 7, Structure of Immunoglobulins.
Written by Zainab Riaz
Zainab Riaz completed her Master degree in Zoology from Fatimah Jinnah University in Pakistan and is currently pursuing a Doctor of Philosophy in Zoology at University of Lahore in Pakistan.
Recent Posts
-
Growth Factors Can Cooperate to Promote Tumorigenesis
Introduction Tumorigenesis, the process by which normal cells transform into cancer cells …3rd May 2024 -
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …29th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024