Unraveling the Complexities of Antibodies: Light Chains, Heavy Chains, Constant Regions, and Tumor-Associated Antigens
Antibodies, also known as immunoglobulins, play a pivotal role in the immune system's ability to recognize and neutralize pathogens. This comprehensive article delves into the structural and functional nuances of antibodies, focusing on the antibody light chain, tumor-associated antigens, constant regions of antibodies, and the interplay between heavy and light chains. By exploring these components in detail, we aim to enhance understanding of their significance in immunology and their implications in cancer research.
Contents
1. Introduction to Antibodies
2. Structure of Antibodies
- Heavy Chain and Light Chain
- Constant Region of Antibody
3. Antibody Light Chain
- Types of Light Chains
- Role and Function
4. Tumor-Associated Antigens
- Definition and Significance
- Examples and Clinical Relevance
5. Heavy Chain and Light Chain: A Synergistic Duo
- Molecular Interaction
- Functional Implications
6. Constant Region of Antibody
- Characteristics and Functions
- Clinical Applications
7.Conclusion
8.References
1. Introduction to Antibodies
Antibodies, also known as immunoglobulins, are pivotal elements of the adaptive immune system, meticulously crafted to identify and neutralize foreign invaders such as viruses, bacteria, and other pathogens. These Y-shaped proteins are synthesized by B cells, a type of white blood cell, in response to the detection of antigens, which are specific molecules or molecular structures, often found on the surfaces of pathogens. The unique architecture and biochemical composition of antibodies enable them to bind to antigens with high specificity, thus playing a critical role in the immune system's ability to remember and attack foreign molecules.
2. Structure of Antibodies
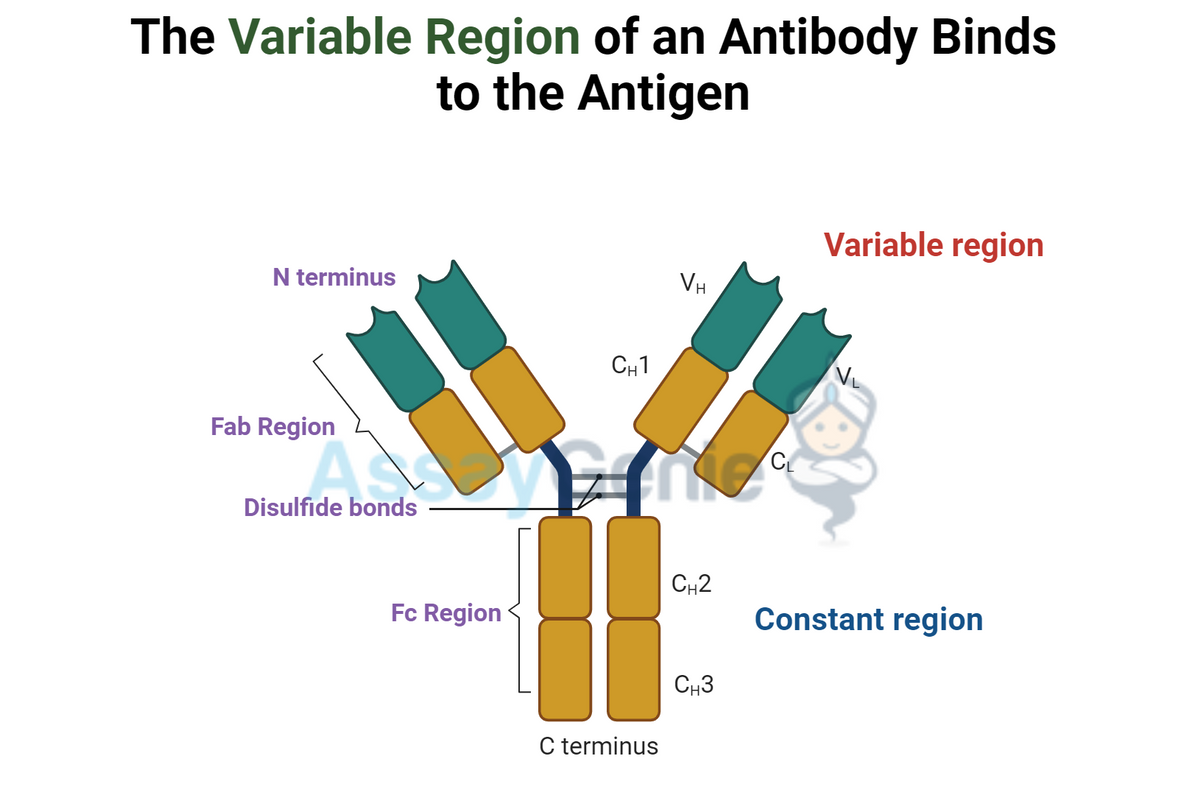
Heavy Chain and Light Chain
The antibody molecule is bifurcated into two primary regions: the Fab (antigen-binding) region and the Fc (constant) region. The Fab region is responsible for antigen recognition, whereas the Fc region mediates interactions with cell surface receptors and the complement system, influencing the immune response.
The heavy and light chains of an antibody are the building blocks that define its unique Y-shaped architecture. Each light chain is composed of one variable (VL) and one constant (CL) domain, whereas each heavy chain contains one variable (VH) domain and three to four constant (CH) domains, depending on the antibody class. The variable domains of the heavy and light chains together form the Fab region of the antibody.
The Fab Region: Key to Antigen Recognition
The Fab (fragment antigen-binding) region is at the forefront of the antibody's defense strategy, responsible for the specific recognition and binding of antigens. This region comprises the variable domains from both the heavy and light chains (VH and VL), which together create a unique antigen-binding site. The variability in the amino acid sequences of these domains allows for a diverse repertoire of antibodies, each capable of binding to a specific antigen epitope. This specificity is fundamental to the adaptive immune system's ability to target and neutralize an incredibly wide array of pathogens.
The Fc Region: Effector Functions Facilitator
Conversely, the Fc (fragment crystallizable) region, primarily formed by the constant domains of the heavy chains (CH), serves as the antibody's effector domain. This region does not participate in antigen binding but is crucial for mediating interactions with various components of the immune system. The Fc region's structure determines the antibody's isotype (e.g., IgA, IgD, IgE, IgG, and IgM), which in turn influences its role within the immune response. These roles include the recruitment of immune effector cells, activation of the complement cascade, and facilitation of antigen presentation, ultimately leading to the clearance of the antigen from the body.
Constant Region of Antibody: Isotype Determination and Immune Response Modulation
The constant region of an antibody, particularly within the Fc portion, plays a vital role in dictating the antibody's isotype. This region exhibits less variability compared to the variable regions, providing a stable framework for class-specific functions. The isotype of an antibody affects how it interacts with other elements of the immune system, including:
- Complement System Activation: Certain antibody isotypes can initiate the complement cascade, a series of enzymatic reactions that leads to the destruction of the pathogen.
- Transplacental Transfer: The Fc region of IgG antibodies facilitates their transfer across the placenta, providing passive immunity to the fetus.
The constant region's structural and functional properties are integral to the antibody's overall efficacy in immune defense, influencing both the direct neutralization of pathogens and the orchestration of broader immune responses.
Functions of the Constant Region
Binding to Fc Receptors: Fc receptors on immune cells recognize the constant region, facilitating antibody-dependent cellular cytotoxicity (ADCC).
Complement Activation: The constant region can initiate the classical complement pathway, leading to pathogen lysis.
Neonatal Immunity: Certain constant regions allow antibodies to cross the placenta, providing passive immunity to the fetus.
Antibody Light Chain
Antibody molecules, also known as immunoglobulins, are quintessential components of the adaptive immune system. They exhibit remarkable specificity and diversity, enabling them to identify and neutralize a vast array of pathogens and foreign molecules. Central to this capability are two types of light chains, kappa (κ) and lambda (λ), which are present in antibodies in a roughly 2:1 ratio in humans. These light chains, together with the heavy chains, form the antigen-binding sites of an antibody, dictating its unique antigen-binding affinity and specificity. This intricate assembly not only underpins the structural integrity of antibodies but also plays a pivotal role in the immune system's ability to recognize and combat a myriad of antigens.
Types of Light Chains
Kappa (κ) Chains
Kappa chains are the predominant type of light chain found in human antibodies. Each kappa chain consists of a variable (V) and a constant (C) domain. The V domain contributes to the antigen-binding site, while the C domain interacts with other components of the immune system. The genetic locus encoding the kappa chain displays considerable variability, which is a source of the antigen-binding specificity and diversity seen in antibodies. This variability allows for the production of a vast repertoire of antibodies, each capable of targeting a unique antigen.
Lambda (λ) Chains
Lambda chains, though less common than kappa chains, play an equally critical role in the immune response. Similar to kappa chains, lambda chains are composed of variable and constant domains that contribute to the antibody's antigen-binding and effector functions. The lambda chain genes also exhibit significant variability, further contributing to the immune system's ability to generate antibodies with high specificity towards an array of antigens.
Table 1: Comparison of Kappa and Lambda Light Chains
| Feature | Kappa (κ) Chain | Lambda (λ) Chain |
| Distribution | ~60% of antibodies in humans | ~40% of antibodies in humans |
| Genetic Loci | κ locus on chromosome 2 | λ locus on chromosome 22 |
| Chain Length | Slightly shorter | Slightly longer |
Role and Function
Tumor-Associated Antigens: Significance in Cancer Immunology
Tumor-associated antigens (TAAs) are proteins expressed on the surface of tumor cells. They can be recognized by the immune system as targets for antibody-mediated responses.
Understanding the landscape of tumor-associated antigens (TAAs) is essential for advancing cancer immunotherapy. Here, we dive deeper into the three key types of TAAs: overexpressed antigens, mutated antigens, and cancer-testis antigens, providing a concise overview with structured information for clarity.
Types of Tumor-Associated Antigens
Understanding the landscape of tumor-associated antigens (TAAs) is essential for advancing cancer immunotherapy. Here, we dive deeper into the three key types of TAAs:
- Overexpressed antigens
- Mutated antigens
- Cancer-testis antigens,
1. Overexpressed Antigens
Normal cellular proteins that are present at much higher levels in cancer cells compared to their normal counterparts. Their overexpression can contribute to the malignant phenotype of cancer cells, making them targets for immunotherapy.
Examples and Significance
- HER2/neu: Overexpressed in certain types of breast cancer, targeted by monoclonal antibodies (e.g., Trastuzumab).
- CD20: Found on the surface of B cells, targeted in non-Hodgkin lymphoma with Rituximab.
Table 1: Overexpressed Antigens
| Antigen | Cancer Type | Therapeutic Target |
| HER2/neu | Breast cancer | Trastuzumab |
| CD20 | Non-Hodgkin lymphoma | Rituximab |
2. Mutated Antigens
Antigens that arise from mutations in the tumor cell genome. These mutations can lead to the production of abnormal proteins that are recognized by the immune system as foreign, providing a basis for targeted therapy.
Examples and Significance
- EGFRvIII: A variant of the EGF receptor, mutated in a subset of glioblastoma, targeted by vaccine therapy.
- BRAF V600E: Mutation in melanoma, targeted by small molecule inhibitors (e.g., Vemurafenib).
Table 2: Mutated Antigens
| Antigen | Cancer Type | Therapeutic Target |
| EGFRvIII | Glioblastoma | Vaccine therapy |
| BRAF V600E | Melanoma | Vemurafenib |
3. Cancer-Testis Antigens
A class of TAAs normally expressed only in male germline cells (e.g., testis) but aberrantly expressed in various tumors. They are immunogenic and considered promising targets for cancer vaccines and other immunotherapies due to their restricted normal tissue expression.
Examples and Significance
- Janeway, C. A., Travers, P., Walport, M., & Shlomchik, M. J. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. Garland Science.
- Murphy, K., & Weaver, C. (2016). Janeway's Immunobiology. 9th edition. Garland Science.
- Klein, J., & Sato, A. (2000). The HLA system. New England Journal of Medicine, 343(10), 702-709.
- Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252-264.
- Weiner, L. M., Surana, R., & Wang, S. (2010). Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nature Reviews Immunology, 10(5), 317-327.
- Ohashi, P. S. (2002). Tumor immunity and autoimmunity: A balancing act. Nature Immunology, 3(4), 337-339.
- Melief, C. J., & van Hall, T. (2012). Therapeutic cancer vaccines. Journal of Clinical Investigation, 122(10), 3395-3400.
- Coulie, P. G., Van den Eynde, B. J., van der Bruggen, P., & Boon, T. (2014). Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nature Reviews Cancer, 14(2), 135-146.
Written by Zainab Riaz
Zainab Riaz completed her Master degree in Zoology from Fatimah Jinnah University in Pakistan and is currently pursuing a Doctor of Philosophy in Zoology at University of Lahore in Pakistan.
Recent Posts
-
Inflammasome Activation Pathways: A Comprehensive Overview
Inflammasomes are complex intracellular structures that play a pivotal role in the immune respon …25th Apr 2024 -
Illuminating the Multifaceted Role of Acetylation: Bridging Chemistry and Biology Introduction:
Acetylation, a chemical process characterized by the addition of an acetyl functional group t …16th Apr 2024 -
Understanding IgA Test: Importance, Procedure, and Interpretation
The IgA test, also known as immunoglobulin A test, is a diagnostic tool used to measure the l …15th Apr 2024