T Cell Types, Activation, Polarization & Function
T Cell Types, Activation, Polarization & Function
A comprehensive guide to T cells!
Introduction
T-cells, vital components of the immune system, encompass various functions and types, including cytotoxic T cells, helper T cells, and regulatory T cells. Their activation and polarization play critical roles in immune responses. Understanding T-cell biology is essential for effective immune defense and therapeutic applications.
Key Takeaways
- T-cells have diverse functions, including cytotoxicity, immune regulation, and coordination.
- Helper T cells activate through interactions with antigen-presenting cells and cytokines.
- T-cell polarization tailors immune responses to specific infections.
- Prolonged antigen stimulation can lead to T-cell exhaustion and reduced function.
- Immunosenescence impacts T-cell function, affecting the aging immune system.
Jump to a section:
Types of T Cells
T-lymphocytes, or T-cells, are a class of adaptive immune effector cells with a wide range of functions and phenotypes. Of these, cytotoxic T cells, T helper cells, and regulatory T cells are among the most commonly studied. Immunophenotyping is used to distinguish T cell types. Different clusters of differentiations— abbreviated CDs— are used to classify cells. Harkening back to the three types of T cells mentioned earlier, cytotoxic T cells are defined by the presence of CD8, helper T cells by CD4, and regulatory T cells by FOXP3. Often the CDs are used when referring to a cell type, eg. FOXP3 T-regs.
Helper T Cells (Th Cells)
Activation of Helper T Cells
Activation occurs when a Helper T Cell comes into contact with either an antigen presenting cell (the macrophage), or more commonly, with one of its surface proteins found on the surface of a pathogen. Helper T cells are activated through interactions with antigen presenting cells, or by cytokines of other immune system cells.
The process of Helper T Cell activation begins when the Helper T Cell interacts with an Antigen Presenting Cell (APC). The APC must first capture and present foreign antigens on its surface. Helper T cells are activated through interactions with Antigen Presenting Cells (APCs). Once the Helper T Cell has come in contact with the APC, it binds to antigen that is displayed on MHC molecules on the APC's cell membrane.
Helper T Cell Receptors
The Helper T cell has receptors on its surface that will bind the antigen only once it binds to an MHC molecule on the APC's cell membrane. Helper T Cells have receptor proteins that allow them to receive information about the external environment of a cell, thereby allowing Helper T Cell Activation to begin.
Helper T cells are activated through interactions with Antigen Presenting Cells (APCs). Helper T cells are activated when they detect cytokines produced by other immune system cells.
CD4+ Helper T Cells
CD4+ ‘helper’ T cells interact with the MHC Class II molecules present on professional antigen presenting cells (‘APC’s). These important T cells serve to bridge the gap between innate and adaptive immunity. Th cells can adopt different phenotypes which can act to heighten or suppress an immune response. Once activated by their cognate antigen and with the correct co-stimulatory signals, mature naive Th cells can proliferate with the help of IL-2, and differentiate into pro-inflammatory Th1 cells or anti-inflammatory Th2 cells depending on the local cytokine environment.
Once Th cells begin producing cytokines, they can be powerful drivers of pro- or anti-inflammatory responses. The cytokines which they produce can act to recruit other immune cells or conversely to knock down the expression of cytokine receptors. Ultimately, many cytokines are pleiotropic in nature and their function is dependent on the local environment and context of their release.
Th1 Helper T Cells
IFNγ and other pro-inflammatory cytokines encourage the differentiation of Th cells into the Th1 phenotype. Th1 cells can bolster the cellular response to pathogens, notably against intracellular bacteria. Th1 cells produce large quantities of interferon gamma and IL-2. They can recruit macrophages to phagocytose intracellular bacteria and CD8+ cytotoxic T cells to induce apoptosis in infected cells.
Th2 Helper T Cells
Anti-inflammatory cytokines like IL-10 and IL-4 drive Th cells to differentiate into the immunosuppressive Th2 phenotype. The extracellular, humoral response is affected by Th2 cells. Th2 cells can act on B cells to induce the production of antibodies as well as the differentiation into memory B cells. They are able to recruit eosinophils and mast cells as well via the production of such cytokines as IL-4, IL-6, IL-10 and IL-13.
Th17 Cells
Th17 cells are capable of producing large quantities of IL-17, a generally proinflammatory cytokine. Th17 cells excel in combating extracellular pathogens and fungi, but can also play a pathogenic role in certain conditions. Th17 cells in the gut have been implicated in IBS and other disruptions to healthy gut function. Th17 cells have a crucial role in protecting mucosal barriers, and recent evidence suggests their function can be regulatory in nature as well as inflammatory.
Th cells can differentiate into Th17 cells when they are exposed to TGF-β, IL-6, IL-21, and IL-23. Higher quantities of IL-6 and TGF-beta can give rise to Th17 cells with a more regulatory phenotype, whereas IL-23 and IL-1 can engender the differentiation of pro-inflammatory Th17 cells. IL-17, which is produced by both variants of Th17 cells, can act on innate immune cells and epithelial cells found in the mucosal barriers which these cells protect.
Cytotoxic T Cells
Natural Killer T Cells (NKT)
Natural Killer T cells (NKTs) are a unique and relatively rare subpopulation of immune cells with characteristics of both natural killer and T lymphocytes. They express an αβ T-cell receptor, as is common in most T cells, but they also bear NK markers like NK1.1. Generally they are CD3+ CD56+ positive, and these two CD are commonly used as NKT markers.
NKT cells can quickly mount an immune response when they encounter DAMPS or inflammatory cytokines. Once NKTs are activated, they are able to exert their effector function via B and T cell activation, DC activation, macrophage recruitment, and NK transactivation. NKTs are capable of secreting large quantities of pro-inflammatory cytokines like IFN-gamma, IL-2, GM-CSF, and TNF-alpha.
CD4+ T Cells
CD4+ T helper cells are an important bridge between innate and adaptive immunity. These cells facilitate the production of antibodies by presenting antigens to B cells. T helper cells are further subdivided by their specific roles, markers, and secreted cytokines (listed below).
CD8+ Cytotoxic T Cells
Cytotoxic CD8+ T lymphocytes are powerful effector cells capable of directly killing abnormal cells by inducing apoptosis. These ‘killer’ T cells interact with MHC Class I, a complex present on all nucleated cells which offers a sample of the proteins which that cell is producing. CD8+ T cells induce apoptosis via perforin/granzyme or FAS ligand pathways. If a killer T cell encounters its cognate antigen on a host cell, it can induce apoptosis and release IL-2 to stimulate proliferation and clonal expansion of T cells with the same antigen specificity.
Regulatory T Cells
Regulatory T cells, often shortened to T regs, are lymphocytes which act to control the immune response. A common name for T reg cells are suppressor T cells, called such because they are able to suppress the immune response and cause the resolution of inflammation. T regulatory cells serve a crucial function in modulating the strength and duration of an immune response. Recently, T regs have been the focus of considerable research attention for their potentially therapeutic roles in cancer, pathogen clearance, and autoimmune disease.
The most common marker for regulatory T cells is FOXP3, which acts as a regulator of transcription. Factors associated with T reg cells are listed below.
FOXP3+ T Reg Cells
Regulatory T cells, commonly called Tregs, are capable of down regulating immune responses. These cells are able to exert anti-inflammatory effects on effector lymphocytes. Under homeostatic conditions, a Treg cell would act as a failsafe in case an autoreactive T cell was able to evade negative selection in the thymus. The ability to ‘turn off’ cytotoxic T cells which react to self-antigens ensures that the adaptive immune system does not unduly destroy healthy cells.
Tregs are often distinguished by their expression of FOXP3, which sets them apart from CD4+ T helper cells with other functions. FOXP3+ T regulatory cells are related to CD4+ Th cells, but ultimately distinct in function and morphology.
Regulatory T cells are capable of producing anti-inflammatory cytokines such as IL-10 and TGF-beta. Additionally, Tregs can also cause other cells to produce IL-10. Tregs can also act directly on effector T cells by inducing apoptosis through granzymes. Tregs are sensitive to IL-2, a cytokine which is released by effector cells and acts upon them, and thus a proxy marker of T cell activity. In areas with a high concentration of IL-2, T regs can bind IL-2 and produce suppressive cytokines to combat the effects of T cell activation.
In cancer, T regulatory cells can create a tolerogenic microenvironment for tumours to develop. Their inherent immunosuppressive function can be co opted by emerging cancerous cells to allow for further growth and immune knockdown.
Gamma Delta T Cells (γδ T)
γδ T cells are a relatively uncommon variant of lymphocytes which bear a T cell receptor composed of unique glycoproteins. Rather than the standard heterodimeric α and β chain TCR, γδ T cells express a TCR with γ and δ domains. These cells are most common in the gut, where they may play a role in maintaining the mucosal barrier. γδ T cells are thought to be activated independently of MHC interaction, making them distinct from their traditional AB counterparts. Because they are not bound by MHC classes, γδ T cells have been the subject of considerable therapeutic research interest, notably for cancer treatment.
CAR-T Cells
Chimeric antigen receptor T cells, called CAR-T cells, are a genetically engineered cellular therapy which combines the cytotoxic power of T cells with unique cancer antigen specificity. These therapies fuse autologous T cells from a patient with a chimeric receptor that is designed to react solely with antigens present on cancerous cells. CAR-T therapy has been extremely successful in the treatment of blood cancers, such as B-cell acute lymphoblastic leukemia (ALL). An ongoing challenge with this therapy is the penetrative and proliferation potential of CAR-T cells over time. Prolonged exposure to the immunosuppressive tumour microenvironment which solid tumours generate can reduce the efficacy of local immune cells. Thus, combinations of cytokines which encourage proliferation (such as IL-2) are currently being explored.
The chimeric antigen receptors which CAR-T cells express have been improved over time. In addition to the variable heavy and light chain antigen binding domain, current fourth generation CARS have a CD3ζ and CD28 co-stimulatory region which aids in cytokine signalling (notably via IL-12).
T Cell Development
T cells are derived from common lymphoid progenitor cells. Common lymphoid progenitor cells can give rise to T cells, NK cells, and B cells. When these progenitors arrive at the thymus, the major site of T cell development, they do not express CD4+ or CD8+ but are CD25+. These thymic progenitors will undergo the differentiation and selection processes which will ultimately make them functional T cells.
Thymocytes, as these potential T cells are called, undergo VDJ recombination to generate unique T cell receptors. This process is mediated by the genes RAG1 and RAG2. Once a functional TCR has been formed, the thymocytes begin to express both CD8 and CD4.
Next, all potential T cells undergo two stringent selection processes. Together, both of these selection rounds ensure that a T cell is not autoreactive yet can bind appropriately to MHC Class I or II. First, thymocytes migrate to the cortex and undergo positive selection. If the cells can interact with MHC I or II, they will receive a survival signal. If not, they will be left to die by apoptosis. This ensures that all T cells will be able to ‘see’ the antigens which APCs present. This process is called positive selection because it requires the addition (+) of a factor to ensure survival. If a cell interacts with MHC Class I it will downregulate CD4 and become a CD8+ cell. If the thymocyte can interact with MHC Class II, it will downregulate CD8 to become a CD4+ cell.
After positive selection is complete, negative selection occurs at the medulla border. Here, the AIRE gene allows for epithelial cells to express self proteins from different regions of the body. Thymocytes are tested for autoreactivity; if the cell reacts strongly with a self antigen, it is destroyed. The only exception is in the case of Tregs, which are allowed to survive in certain cases where self reactivity is shown.
Only a small fraction of thymocytes survive the double selection process. Those that successfully complete thymic development are mature naive T cells and exit the thymus.
T Cell Polarization
T cell polarization is the process by which T cells become specialized to fight specific types of infections. T cell polarization is controlled by a complex series of signaling events that occur between the T cell and the infection-causing agent. These signaling events result in the activation of specific T cell receptors, which then activate different signaling pathways that lead to the production of different types of T cells.
T helper cells are a type of T cell that helps to regulate the immune response. T helper cells produce chemicals that help to activate other T cells and promote the production of antibodies. T regulatory cells are a type of T cell that helps to suppress the immune response. T regulatory cells produce chemicals that help to inhibit the activity of other T cells. T cell polarization is a critical process in the development of an effective immune response.
T cell polarization allows the body to produce the right type of T cell for the specific infection that is being fought. T cell polarization also helps to ensure that the immune response is appropriately regulated and does not become excessive. T cell polarization is a complex process, but it is essential for the proper functioning of the immune system.
T Cell Expansion & Negative Selection
T cells go through a process called expansion when they first encounter an antigen, which is a substance that the body views as foreign. During expansion, the T cells multiply rapidly so that there are enough of them to mount an effective response to the invader. After the initial response is over, the T cells then go through a process called selection, during which only the T cells that are specific for that particular antigen are retained. The rest of the T cells die off. This ensures that the body has a population of T cells that is specific for each type of invader it may encounter.
T Cell Cytokine Signalling
Cytokine secretion and receptivity are crucial for the proper function of T cells and the immune system as a whole. T cells are major players in host defence and are protective against pathogen invasion and cancer. T cells have the ability to generate a robust and destructive inflammatory response which can act to clear abnormal cells that are detrimental to the survival of an organism. This same ability can make T cells a driver of autoimmune disease, and thus other suppressive cells— like T regulatory cells— are important in maintaining balance in immune response and resolution.
Cytokines such as IL-1 are pro-inflammatory and implicated in T cell activation. IL-2 is an important cytokine for the continued survival and proliferation of T cells. Anti-inflammatory cytokines like IL-10 and TGF-β are implicated in the resolution of the immune response and T reg function. A host of other cytokines contribute to T cell function, activation, and proliferation.
Lymphokine Release
Helper T Cells also release chemicals known as lymphokines which in turn activate the macrophage, further enhancing Helper T Cell activation. Helper T Cells are activated through interactions with Antigen Presenting Cells (APCs). Helper T cells are activated when they detect cytokines produced by other immune system cells.
Helper T-cells are a type of white blood cell that play critical roles in the immune system. Helper T cells are also called CD4 positive T cells because they have a protein on their surfaces called CD4. Helper T-cells help coordinate the body's response to foreign invaders by recruiting and activating other white blood cells such as macrophages, Natural Killer Cells, dendritic cells, B-cells and cytotoxic T cells. Helper T cells can recognize infected or damaged host cells as well as altered self-molecules (such as cancerous cells). Helper T cell is activated when it detects either an antigen within the cell or small molecules released by bacteria or virus infected host cells.
Once Helper T cells recognize the abnormal cells, they release cytokines and activate other Helper T Cells as well as cytotoxic T cells to destroy the infected cell. Helper T cells also regulate B-cell responses and CD8+ Cytotoxic T Cell responses.
Contact independent activation of Helper T Cells
Contact independent activation happens when Helper T Cells have already encountered Helper T Cell specific antigens on MHC II molecules on APCs Helper T cells are always activated when they bind to MHC II molecules Helper T Cells effector functions are triggered by the process of cross-presentation, which involves Helper T Cells being activated by dendritic cell APCs Helper T Cells that have been activated will begin making proteins called cytokines, which are essentially chemical messengers. These cytokines signal other white blood cells to perform specific tasks.
T Cell Exhaustion
T cell exhaustion occurs when effector T cells are no longer able to respond appropriately after prolonged antigen stimulation. Generally, the phenomenon is associated with cytotoxic CD8+ T cells. Hallmarks of exhaustion include:
1) Decreased ability to proliferate
2) Reduced cytotoxic ability
3) Decrease in cytokine production
4) Increased expression of inhibitory checkpoints
T cell exhaustion can be induced in cases of viral infection or cancer. Generally, it is thought to exist on a graded scale rather than as a binary. The magnitude and duration of antigen exposure is thought to be a factor in the onset time and severity of T cell exhaustion. T cell exhaustion assays can be used to measure the extent of effector change in a population of T cells. CD4+ T cells have also been shown to develop an exhaustion-like response following chronic unresolved antigen exposure.
Immunosenescence in T Cells
Immunosenescence is the process of immune system dysfunction caused primarily by aging. The adaptive immune system is more strongly affected by immunosenescence than the innate immune system, but both branches show decreased efficacy as ontogenic time progresses.
T cells are a key mediator of the immune response, both as direct effector and as coordinators. As the body ages, naive T cells become rarer and less robust. Ultimately, a decrease in effective leukocyte/lymphocyte count causes a decrease in the immune response.
An increase in CD28- memory T cells can contribute to the phenomenon, as T cells lacking CD28 cannot receive proper activation signals. This can cause anergy or a decrease in the production of appropriate cytokines.
T Cell Antigen Recognition
T cells are activated when the T cell receptor (TCR) binds its cognate antigen in the presence of other co-stimulatory factors. Each TCR is able to bind one unique antigen, and an entire population of T cells within one organism is able to bind millions of potential antigens. Upon TCR activation with the appropriate co-signals, T cells undergo expansion. T cells express either CD4 or CD8, which interact with MHC class II or MHC class I, respectively, on antigen presenting cells. The MHC 'presents' the antigen to the T cell while the APC also generates the secondary cytokine and CD28 signals necessary for proper T cell activation.. In order to avoid anergy, generally all three signals must be present.
Activation Signals:
-
- TCR binds to MHC/antigen complex
- CD28 (expressed on T cells) binds with CD80/86 (B7-1 and B7-2) on the antigen presenting cell.
- Cytokines
T Cells in Cytokine Release Syndrome
T Cells have been implicated in cytokine release syndrome. As cells with a strong potential to recruit and activate immune factors, such as cytokines, they have also become a promising target for therapeutic intervention. IL-6 is a primary driving cytokine in CRS, and this cytokine is a downstream product of T cell activation and subsequent immune cell recruitment. IL-6 has been the target of considerable therapeutic interest. At present, IL-6 antibodies such as tocilizumab are used to treat cytokine release syndrome (CRS), including in cases of COVID-19.
T cell Assays
Popular T Cell Assays
Types of T cell Assays
T Cell Assays
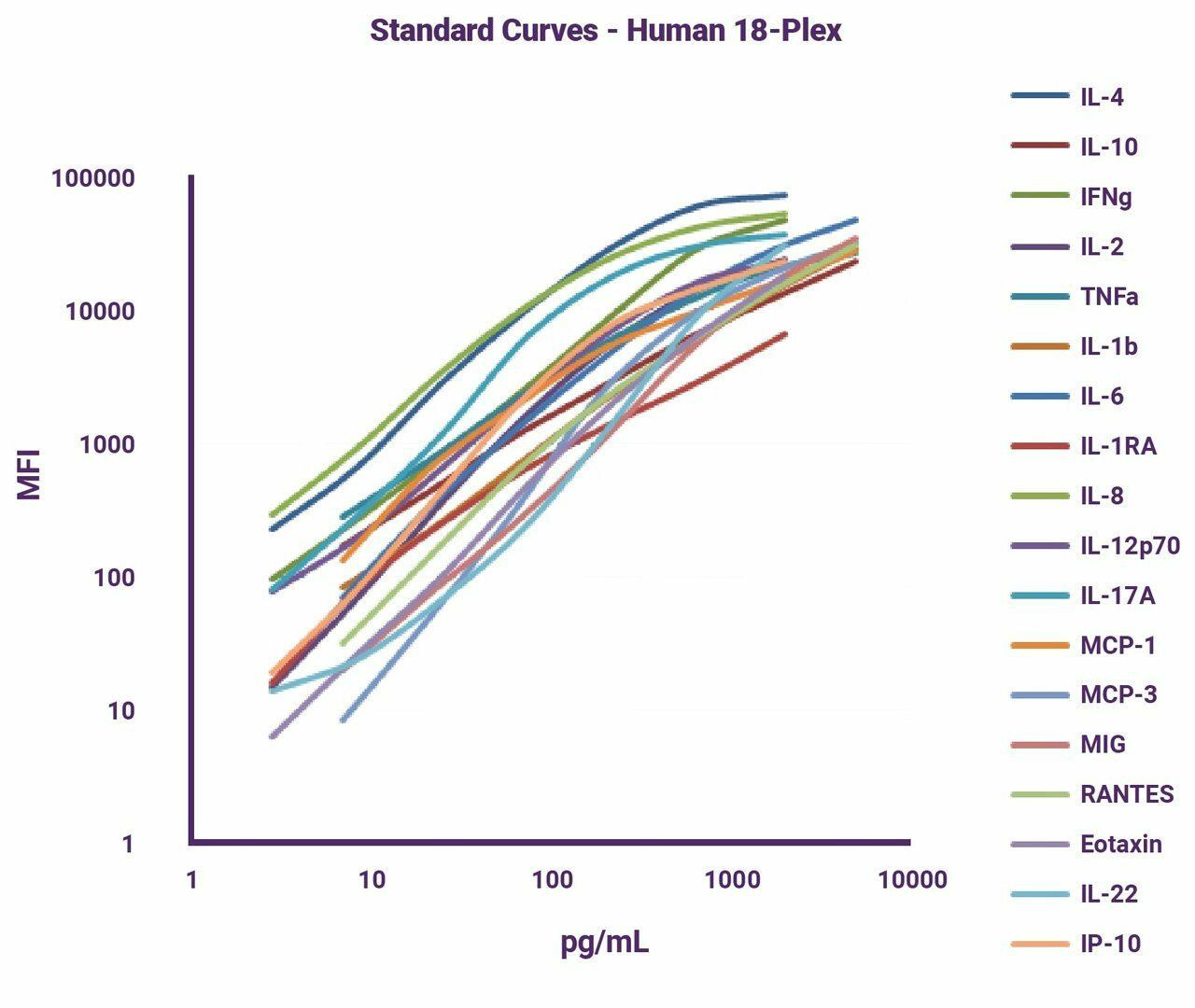
T-cell infiltration is the ability for a cell to travel inside of solid tissue to enact effector functions. Especially in the tumour microenvironment, it is crucial that anti-tumour cells be able to effectively penetrate the solid mass of malignant cells. Infiltration is part of the reason chimeric antigen receptor T-cell and autologous T-cell therapy (CAR-T/ACT) has been so successful in blood cancers but less advantageous in solid tumours. In order for a cell to enact its effector function—to kill— it must be in close physical proximity so that it can engage with the dysfunctional cell via direct contact. T-cell infiltration assays measure the level of CD3, a TCR co-receptor, to determine the presence of T-cells in a sample. To learn more about this useful marker for measuring infiltration, check out the protocol for our CD3e human ELISA kit.
T Cell Exhaustion Assays
T-cell exhaustion is a dysfunction caused by the constant stimulation of T-cells, often due to a chronically unresolved inflammatory response. T-cell exhaustion assays measure the level of inhibitory receptors present on the cell surface of CD4+ and CD8+ cells, which often are upregulated during the exhaustion phase of the T-cell life cycle. Common markers include PD-1 and CTLA-4. The causes, course, and reversal of T-cell exhaustion is an area of great interest in the scientific community today. This is, in part, because of the role that CAR-T and ACT have come to play in the treatment of certain cancers, and the growing interest in the complex role of the immune system in cancer therapy.
T Cell Cytotoxicity Assays
T-cell cytotoxicity is the ability of a T-cell to directly cause the death of another cell. T-cell cytotoxicity assays measure the level of cells bearing CD8a, the glycoprotein which interacts with the MHC Class 1 present on most nucleated cells. CD8+ cytotoxic T-cells are thought to mediate cytotoxicity. Our human CD8 alpha/ CD8A ELISA kit allows for the measurement of this type of effector cell in humans. Similarly, ADCC (antibody-dependent cell-mediated cytotoxicity) is a way to determine if a helper T-cell response has allowed for antibody production via B-cell to plasma B cell differentiation. Our ADCC assay kit can help to identify effector function by labelling late apoptotic and necrotic target cells.
T Cell Proliferation Assays
T-cell proliferation refers to the ability of a T-cell to divide in vivo. Because of the restrictive tumour microenvironment, nutrient and oxygen availability is often low. Such conditions can culminate in the failure of lymphocytes to survive once they reach their target tissues. T-cell proliferation assays measure the division response of T-cells when proliferation-stimulating factors are used. CD7 is an early marker on T-cells, present from the pre-thymic stage all the way through to maturity. Check out this kit to learn more (Source: Atlas of Hematopathology (Second Edition), 2018, Pages 29-56).
Jurkat Cell Line
The Jurkat T-cell line (often referred to as JM) is a leukaemic T-cell line that was established in 1977 from the peripheral blood of a 14 year old boy with acute lymphoblastic leukaemia (ALL) [Schneider et al., 1977]. The cells were first used in culture in antibody screens to identify T lymphocytes at the Fred Hutchinson Cancer Research Center, Seattle, USA [Martin et al.,1981], as such early publications refer to Jurkat cells as Jurkat-FHCRC cells [Weiss et al., 1984].
However, it was discovered that Jurkat-FHCRC cells were heavily contaminated with mycoplasma and the removal of the mycoplasma infection from the cells resulted in the creation of the Jurkat E6-1 clone, which is the most common Jurkat cell line in use today. Initial interest in Jurkat cells arose from studies on T-cell activation through interleukin-2 production, as such a number of T-cell receptor signalling mutants have been created, such as the CD45-deficient line J45.01 [Koretzky et al., 1991] and the lymphocyte-specific protein tyrosine kinase (Lck)-deficient line J.Cam1 [Goldsmith and Weiss, 1987].
The number of publications that include ‘Jurkat’ as a keyword rose by over 50-fold from the cell line inception in the early 1980s to 2003 [Abraham and Weiss, 2004] and currently Jurkat cells are used to study acute T-cell leukaemia, T-cell signalling, the expression of cellular receptors susceptible to viral entry and to examine the susceptibility of cancers to chemotherapeutic drugs and radiation.
Written by Sean Mac Fhearraigh
Seán Mac Fhearraigh PhD is a co-founder of Assay Genie. Seán carried out his undergraduate degree in Genetics at Trinity College Dublin, followed by a PhD at University College Dublin. He carried out a post-doc at the Department of Genetics, University of Cambridge. Seán is now Chief Technical Officer at Assay Genie.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026