Unlocking Cellular Adaptation: The HIF Enhancer Pathway and Its Implications in Hypoxia Response
Hypoxia, or low oxygen levels, poses a significant challenge to cells, necessitating rapid and efficient adaptation mechanisms. The Hypoxia-Inducible Factor (HIF) pathway is a well-known regulator of cellular responses to hypoxia, orchestrating the expression of genes involved in angiogenesis, erythropoiesis, and glycolysis. It plays a pivotal role in cellular adaptation to low oxygen conditions, ensuring survival and homeostasis in diverse physiological and pathological settings. Recent studies have uncovered an additional layer of complexity in HIF regulation, known as the HIF enhancer pathway. This article explores the mechanisms, regulation, and significance of the HIF enhancer pathway in cellular responses to hypoxia.
Mechanisms of the HIF Enhancer Pathway:
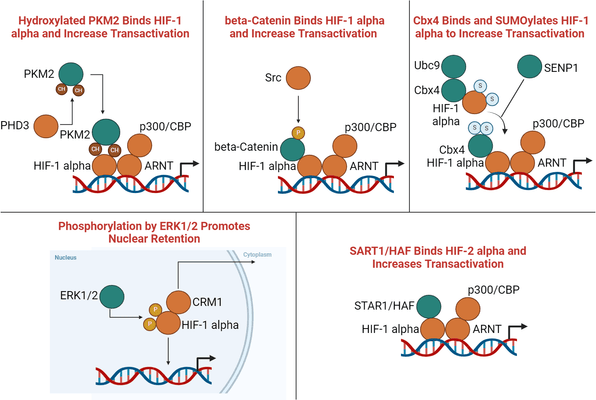
The HIF enhancer pathway operates through a series of molecular events that amplify the HIF response under hypoxic conditions. One key player in this pathway is the HIF prolyl hydroxylase (PHD), which traditionally acts as a negative regulator of HIF by targeting it for degradation in normoxia. However, under hypoxic conditions, the HIF enhancer pathway is activated, leading to increased stability and activity of HIF.
The enhanced stability of HIF in hypoxia is attributed to the inhibition of PHD activity by factors such as reactive oxygen species (ROS) and metabolic intermediates. Additionally, recent studies have identified novel proteins that directly interact with HIF, shielding it from degradation. These interactions contribute to the prolonged presence of active HIF in the nucleus, promoting the transcription of downstream target genes.
Mechanism of the HIF Enhancer Pathway under Hypoxic Conditions
Regulation of the HIF Enhancer Pathway:
The HIF enhancer pathway is tightly regulated to ensure a balanced cellular response to hypoxia. Several signaling pathways converge to modulate the activity of key components in this pathway. For instance, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is known for its involvement in cell survival, has been shown to activate the HIF enhancer pathway under hypoxic conditions. Furthermore, hypoxia-induced changes in cellular metabolism, including alterations in tricarboxylic acid (TCA) cycle intermediates, can impact the HIF enhancer pathway.
Epigenetic modifications also play a crucial role in regulating the HIF enhancer pathway. Recent studies have demonstrated that histone acetylation and DNA methylation at specific loci can modulate the accessibility of enhancers associated with HIF target genes. These findings highlight the intricate interplay between genetic and epigenetic mechanisms in fine-tuning the HIF enhancer pathway.
Significance of the HIF Enhancer Pathway:
Understanding the HIF enhancer pathway has far-reaching implications for various fields, including cancer biology, cardiovascular research, and regenerative medicine. In cancer, for instance, the dysregulation of the HIF pathway is a hallmark of tumor progression. The identification of the HIF enhancer pathway provides novel therapeutic targets for intervention, aiming to disrupt the delicate balance that sustains cancer cell survival in hypoxic microenvironments.
In cardiovascular research, the HIF enhancer pathway has been linked to ischemic preconditioning, a phenomenon where brief episodes of hypoxia protect cells from subsequent severe hypoxic insults. Unraveling the intricacies of the HIF enhancer pathway may unveil new strategies to harness the therapeutic potential of ischemic preconditioning for cardiovascular diseases.
Implications for Future Therapies
The HIF enhancer pathway represents a new frontier in the understanding of cellular responses to hypoxia. Its intricate regulatory network and cross-talk with various cellular processes underscore its significance in maintaining cellular homeostasis under challenging conditions. As research in this field progresses, the identification of specific molecular players and the development of targeted interventions hold promise for advancing therapeutic strategies in diseases associated with hypoxia, ultimately improving patient outcomes.
References:
- Semenza, G. L. (2012). Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in pharmacological sciences, 33(4), 207-214.
- Majmundar, A. J., Wong, W. J., & Simon, M. C. (2010). Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell, 40(2), 294-309.
- Kaelin Jr, W. G., & Ratcliffe, P. J. (2008). Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular cell, 30(4), 393-402.
- Lee, K. E., & Simon, M. C. (2015). From stem cells to cancer stem cells: HIF takes the stage. Current opinion in cell biology, 37, 24-29.
- Wenger, R. H., & Stiehl, D. P. (2011). Camenisch G. Integration of oxygen signaling at the consensus HRE. Science's STKE, 2005(306), re12.
- Schofield, C. J., & Ratcliffe, P. J. (2004). Oxygen sensing by HIF hydroxylases. Nature reviews. Molecular cell biology, 5(5), 343-354.
- Taylor, C. T. (2008). Mitochondria and cellular oxygen sensing in the HIF pathway. Biochemical Journal, 409(1), 19-26.
- Batie, M., Frost, J., Frost, M., Wilson, J. W., Schofield, P., & Rocha, S. (2019). Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science, 363(6432), 1222-1226.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026