Cyclins and Cyclin Dependent Kinase – Review
Cyclins and CDKs are central to cell cycle control, with specific cyclin-CDK pairs regulating different phases, ensuring orderly progression and genomic integrity in cell division.
Key Takeaways:
- Cyclins and CDKs are vital for cell cycle regulation.
- Cyclins A, B, D, E each pair with specific CDKs to control cell cycle phases.
- Cdk1, the prototypical CDK, partners with cyclin B for mitotic entry.
- Cyclin B upregulation and Cdk1 substrates play key roles in mitosis.
- Proteomic analysis reveals extensive Cdk1 substrates in mitosis.
- Positive feedback loops amplify Cdk1/cyclin B activity.
- Cdk1-Plk1 phosphorylation cascade is crucial for spindle assembly and mitosis.
- Cyclin degradation by APC/C-Cdc20 complex signals anaphase onset.
Introduction
Cyclins, a family of proteins, play a crucial role in regulating the cell cycle by acting as essential activating co-factors for cyclin-dependent kinases (CDKs). These proteins, although non-enzymatic themselves, form a dynamic partnership with CDKs, enabling the precise control of various cell cycle transitions. Initially discovered by Timothy Hunt in 1982 while studying the cell cycle of sea urchins, cyclins were later recognized as a fundamental mechanism for regulating the cell cycle in eukaryotes. By binding to CDKs, cyclins facilitate the formation of CDK/cyclin complexes, which effectively target specific substrates for phosphorylation by the active kinase. This intricate interaction between cyclins and CDKs orchestrates the sequential progression of the cell cycle, ensuring accurate cell division and maintenance of genomic integrity.
Serine/threonine kinases known as cyclin-dependent kinases (CDKs) depend on the cyclin regulatory component for their function. Based on the kinase domain sequence, CDKs, glycogen synthase kinase-3 beta (Gsk3), members of the dual-specificity tyrosine-regulated kinase (DYRK) family, and CDK-like kinases are all included in the CMGC group of kinases. While CDKs are distinguished by dependence on different protein subunits that supply extra sequences necessary for enzymatic activity, similar kinases like MAPKs are characterized by substrate specificity being supplied by docking sites separated from the catalytic region. Recent renaming of the proteins in this family as Cdk1 through Cdk20 has made it easier to identify and analyze CDKs.
Cyclins A, B, D, E
The regulation of the cell cycle in humans relies on the expression and activity of several key cyclins, namely cyclins A, B, D, and E. Each of these cyclins forms a specific partnership with its corresponding cyclin-dependent kinase (CDK)-binding partner, ensuring precise control over the progression of the cell cycle. Among these cyclins, cyclin B exhibits a unique relationship with Cdk1. Together, they form a bipartite complex, with cyclin B acting as a crucial activator for Cdk1. This complex plays a pivotal role in triggering and coordinating critical cell cycle events, such as mitosis. By inducing the activation of Cdk1, cyclin B drives the transition from the G2 phase to mitosis, ensuring proper chromosome segregation and cell division. This elegant interplay between cyclin B and Cdk1 highlights the intricate nature of cyclin-CDK complexes and their indispensable contributions to the cell cycle regulation.
Cell cycle Regulators
Cyclin dependent kinase 1 (Cdk1)/Cyclin B complex
As the archetypal member of the cyclin-dependent kinase (CDK) protein family, Cdk1 plays a pivotal role in orchestrating the progression of the cell cycle. Particularly during mitosis, Cdk1 forms a complex with cyclin B, known as the Cdk1/cyclin B mitotic kinase complex. This complex serves as a key regulator of mitotic entry, ensuring the proper timing and coordination of crucial events. Interestingly, Cdk1 and cyclin B were initially discovered as components of a multiprotein complex called maturation promoting factor (MPF) in Xenopus oocytes. The identification of MPF marked a significant milestone in understanding the molecular mechanisms governing cell cycle progression. The Cdk1/cyclin B mitotic kinase complex, with its intricate interplay and precise regulation, holds the responsibility of initiating and executing mitosis, including processes such as chromosome condensation, spindle formation, and cell division. This pivotal role of Cdk1 further highlights its importance as a master regulator of the cell cycle.
Cyclin B upregulation
During the G2 phase of the cell cycle, the levels of Cyclin B undergo a significant upregulation in preparation for mitotic entry. This upregulation of Cyclin B is closely associated with both the rapid increase in transcription of Cdk1 and the activation of Cdk1 itself. Cdk1 activation, facilitated by the binding of Cyclin B, plays a crucial role in regulating mitotic entry by initiating key events, such as the breakdown of the nuclear envelope during prophase. This process triggers chromatin condensation, which allows for the efficient segregation of sister chromatids by the mitotic spindles. The coordinated action of Cyclin B and Cdk1 orchestrates the sequential events necessary for proper mitotic progression. By promoting the breakdown of the nuclear envelope and facilitating chromatin condensation, Cyclin B and Cdk1 ensure the accurate separation and distribution of genetic material during cell division. The upregulation of Cyclin B, coupled with the activation of Cdk1, marks the critical transition from the G2 phase to mitosis, ensuring the faithful execution of mitotic processes.
Cdk1 substrates
While many of its substrates remain to be fully identified, studies have shed light on the phosphorylation motifs recognized by Cdk1. It has been determined that Cdk1 phosphorylates substrates at motifs consisting of S/TPXK/R, with the phosphorylation occurring primarily at the serine or threonine residue within this motif. This specific motif serves as a recognition site for Cdk1, allowing it to exert its regulatory function on a wide range of target proteins. Interestingly, in yeast and certain other species, a broader motif of S/TP has been shown to be sufficient for Cdk1 phosphorylation. This suggests a degree of flexibility and divergence in the motifs recognized by Cdk1 across different organisms. The identification and characterization of Cdk1 substrate phosphorylation motifs provide valuable insights into the regulatory mechanisms underlying cell cycle progression and mitotic control. Further exploration of these motifs holds the potential to uncover novel Cdk1 substrates and shed light on additional aspects of the intricate network of cellular signaling pathways involved in cell division.
Proteomic analysis of Cdk1 substrates
The knowledge of the Cdk1 phosphorylation motif has opened doors to proteomic investigations aimed at uncovering previously unknown Cdk1 mitotic substrates. One such study involved a phosphoproteomic investigation of the mitotic spindle, which resulted in the identification of 736 distinct phosphorylation sites occurring within the Cdk1 motif region of various proteins. This comprehensive analysis provided valuable insights into the breadth and diversity of Cdk1-mediated phosphorylation events during mitosis.
Similarly, investigations in S. pombe budding yeast revealed approximately 200 distinct Cdk1 substrate proteins. Interestingly, many of these proteins showed homology to components involved in the SAC (spindle assembly checkpoint) pathway or other aspects of mitotic progression. These findings shed light on the extensive involvement of Cdk1 in regulating critical cellular processes required for accurate cell division and genomic stability.
Notably, a significant discovery emerged from the interplay between the Cdk1 phosphorylation motif and the Plk1 (Polo-like kinase 1) PBD (polo-box domain) recognition motif. It was realized that both motifs require a serine/threonine residue followed by an immediately adjacent proline residue. This discovery unveiled a key mechanism for the regulation of Plk1 activity, as Plk1 is known to act downstream of Cdk1 during mitotic progression. The interplay between these phosphorylation motifs provides a coordinated regulatory mechanism for the precise control of key mitotic events.
These proteomic investigations and the identification of Cdk1 mitotic substrates, along with the interplay with Plk1, contribute to our understanding of the complex network of protein interactions and regulatory mechanisms underlying mitotic progression. Further exploration of these findings holds the potential for uncovering additional players and mechanisms involved in the intricate orchestration of cell division.
Polo-like kinase phosphorylates Cdk1
Cdk1 exhibits the ability to positively regulate its own activity through several distinct feedback loops. These feedback mechanisms contribute to the robustness and stability of Cdk1/cyclin B activity during mitosis. For instance, Cdk1 can phosphorylate and activate proteins involved in promoting its own activity, such as the phosphatase Cdc25. This positive feedback loop amplifies Cdk1/cyclin B activity, resulting in the efficient progression of mitotic events. Additionally, Cdk1 can also exert positive feedback by phosphorylating inhibitory kinases, such as Wee1 and Myt1, leading to their inactivation and further promoting Cdk1/cyclin B activity.
These intricate feedback loops and phosphorylation events involving Cdk1/cyclin B highlight the multifaceted nature of cell cycle regulation. The interplay between transcriptional control, post-translational modifications, and feedback mechanisms ensures the precise activation and coordination of Cdk1/cyclin B activity, ultimately driving the orderly progression of mitotic events.
Cdk1 – Plk1 phosphorylates mitotic spindle
The priming mechanism in Cdk1-Plk1 phosphorylation has revealed numerous specific examples that contribute to the complex spatio-temporal regulation of the mitotic spindle. These phosphorylation events play critical roles in coordinating various aspects of spindle assembly and mitotic progression. One such example involves Nedd1, a component of the γ-tubulin ring complex (γTuRC). Nedd1 is sequentially phosphorylated by Cdk1 and Plk1 during the coordination of spindle assembly. This sequential phosphorylation of Nedd1 facilitates the localization of the γTuRC to the centrosome, enabling the efficient nucleation of microtubules at the microtubule-organizing center (MTOC). Through this mechanism, Cdk1 and Plk1 orchestrate the precise organization and functionality of the mitotic spindle.
Additionally, the sequential Cdk1-Plk1 phosphorylation of BubR1 at the kinetochore regulates the activation of the spindle assembly checkpoint (SAC) pathway. This phosphorylation event serves as a crucial step in transducing a wait-anaphase signal. BubR1, when phosphorylated by Cdk1 and Plk1, undergoes changes that regulate its interaction with other SAC components, ensuring proper chromosome alignment and preventing premature segregation during mitosis. This dynamic regulation of BubR1 by Cdk1 and Plk1 exemplifies the intricate control mechanisms that ensure accurate chromosome segregation.
Mitotic timing
The successful completion of mitosis relies on the tight temporal regulation of mitotic kinases, which govern crucial chromosomal dynamics and facilitate the transition from metaphase to anaphase. It is proposed that a hierarchical activation of three mitotic kinases plays a central role in orchestrating these events. This hierarchy involves the activation of Cdk1, Plk1, and Aurora B kinase, each with distinct functions and specific targets during mitotic progression.
The regulation of mitotic kinases occurs at multiple levels, ensuring precise control over their activity. Firstly, at the transcriptional level, there is a coordinated regulation of gene expression. Studies have shown that the transcription of key mitotic kinases, such as Cdk1, Plk1, and Aurora B, is tightly controlled. Their expression levels are tightly regulated to ensure their availability at the appropriate stages of the cell cycle. This transcriptional regulation contributes to the temporal activation of these kinases and the proper execution of mitotic events.
Additionally, the activity of mitotic kinases is regulated by protein phosphatases that act as their antagonists. These protein phosphatases counteract kinase activity by dephosphorylating specific target proteins. Notably, phosphatases such as Cdc25 and PP2A play critical roles in fine-tuning the activity of mitotic kinases. By opposing kinase activity, these phosphatases contribute to the dynamic regulation of mitotic events and prevent untimely progression.
Cyclins Related Products
Cyclins ELISA Kits
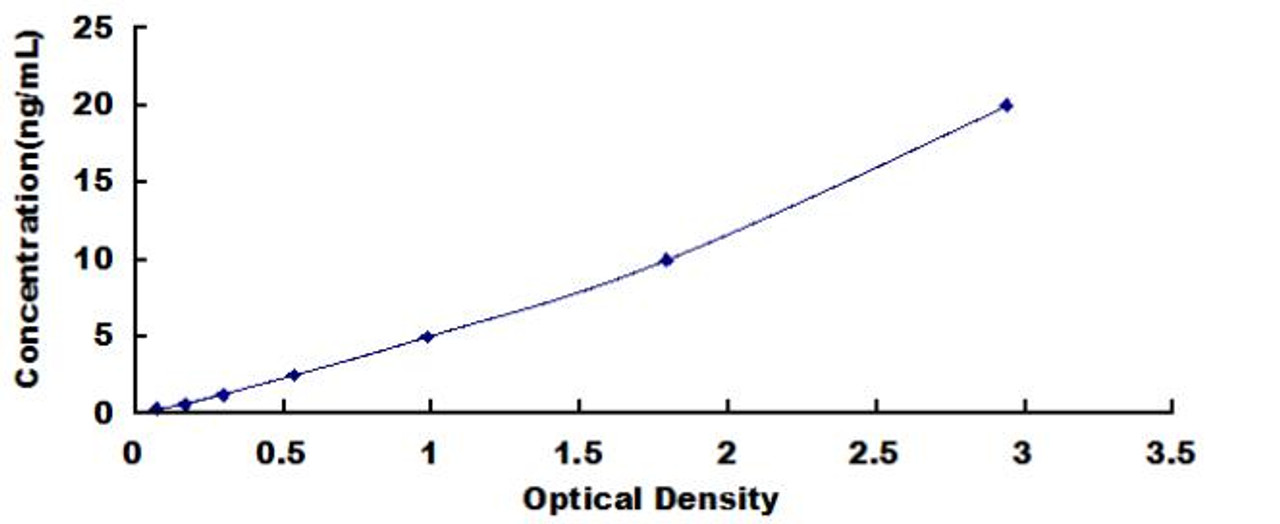
| Human Cyclin B (CCNB) ELISA Kit | |
|---|---|
| Sensitivity | 0.124ng/mL |
| Range | 0.312-20ng/mL |
| ELISA Type | Sandwich |
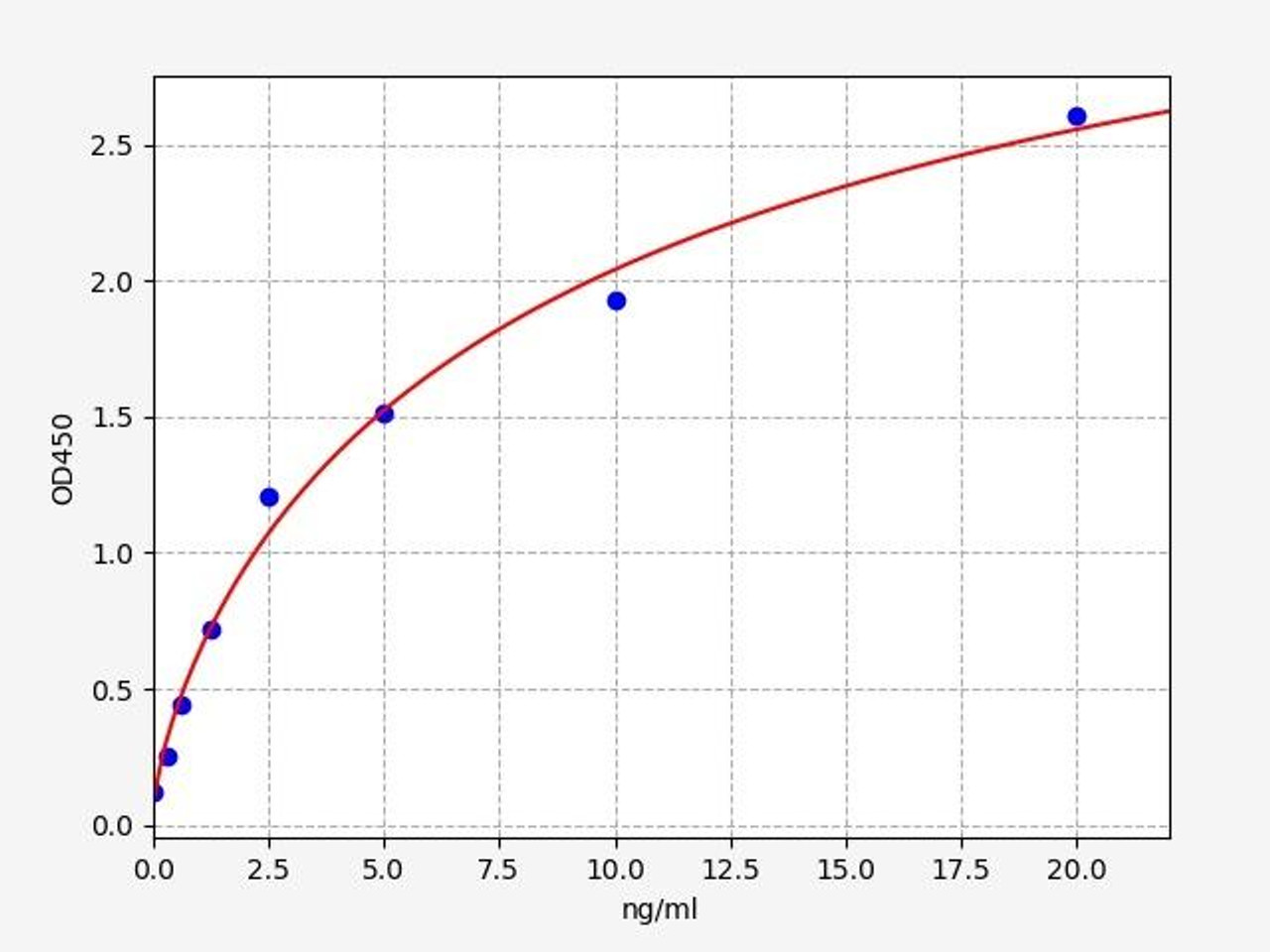
| Human Cyclin D1 ELISA Kit | |
|---|---|
| Sensitivity | 0.188ng/ml |
| Range | 0.313-20ng/ml |
| ELISA Type | Sandwich ELISA, Double Antibody |
| Human Cyclin-dependent kinase 1 (CDK1) ELISA Kit | |
|---|---|
| Size | 96 Assays |
| ELISA Type | Sandwich |
| Reactivity | Humans |
Anaphase progression
As the mitotic cell enters anaphase, a crucial event for the progression of cell division, the degradation of cyclin B is initiated. This degradation is mediated by the Anaphase-Promoting Complex/Cyclosome (APC/C) in a Cdc20-dependent manner, as demonstrated by studies. The targeting of cyclin B for degradation by the APC/C leads to the inactivation of the Cdk1 kinase activity.
The degradation of cyclin B and subsequent inactivation of Cdk1 kinase activity are pivotal for the progression of anaphase and the completion of cytokinesis. By eliminating cyclin B, the APC/C-Cdc20 complex effectively eliminates the activating co-factor required for Cdk1's kinase activity, preventing its sustained function in driving mitotic processes. This cyclin B degradation and Cdk1 inactivation release the inhibitory control over anaphase-promoting events, allowing sister chromatid separation and the subsequent formation of daughter cells during cytokinesis. The degradation of cyclin B and the abrogation of Cdk1 kinase activity mark a critical transition in the cell cycle, enabling the final stages of cell division.
The precise timing and regulation of cyclin B degradation by the APC/C-Cdc20 complex ensure the fidelity of chromosome segregation and the accurate completion of cell division. This degradation-dependent inactivation of Cdk1 kinase activity serves as a key mechanism in coordinating the events of anaphase and cytokinesis, contributing to the maintenance of genomic stability and the generation of two genetically identical daughter cells.
Inner centromere phosphorylation
The inner centromere protein INCENP has been proposed to play a crucial role in the tripartite phosphorylation pathway involved in mitotic progression. Specifically, INCENP is involved in the dynamic interplay between Cdk1, Plk1, and Aurora kinase B, contributing to the precise orchestration of events at the kinetochore during mitosis.
Cdk1 priming phosphorylation of INCENP at threonine-59 and threonine-388 has been shown to be essential for the recruitment of Plk1 to the kinetochore. This priming phosphorylation event primes INCENP, creating docking sites for Plk1, facilitating its localization to the kinetochore. The recruitment of Plk1 to the kinetochore brings it into close proximity with Aurora kinase B, enabling their functional collaboration.
The proximity between Plk1 and Aurora kinase B at the kinetochore, facilitated by INCENP, allows for efficient coordination of their activities. Plk1 plays a crucial role in regulating the activity of Aurora kinase B through phosphorylation events, ensuring proper attachment of microtubules to the kinetochore, correction of erroneous attachments, and the generation of the necessary forces for faithful chromosome segregation.
Phosphastases temporal activation during mitosis
Recent research has shed light on the temporal regulation of protein phosphatases, revealing that their activity is not necessarily constitutively active throughout the cell cycle. Instead, the precise regulation of phosphatases is recognized as crucial for various stages of mitosis. This temporal regulation ensures the tight control and coordination of phosphorylation and dephosphorylation events that drive mitotic progression.
Furthermore, it has been observed that many phosphatases exhibit high activity during the coordination of mitotic exit. This finding presents an attractive therapeutic target for anti-mitotic strategies. Targeting phosphatases involved in coordinating mitotic exit holds promise as a potential therapeutic approach, particularly because therapeutic efficacy would not be compromised by mitotic slippage.
The temporal regulation of protein phosphatases throughout mitosis highlights their importance in balancing the phosphorylation and dephosphorylation events that drive the dynamic changes in cell cycle progression. Understanding the spatiotemporal regulation of phosphatases and their roles in mitotic control provides valuable insights into potential therapeutic strategies for modulating mitotic processes. Targeting phosphatases involved in mitotic exit could offer novel avenues for the development of anti-mitotic therapies with improved efficacy and reduced chances of mitotic slippage.
Written by Pragna Krishnapur
Pragna Krishnapur completed her Bachelor degree in Biotechnology Engineering in Visvesvaraya Technological University before completing her Masters in Biotechnology at University College Dublin.
Recent Posts
-
Exhibition at the University of Oxford's BioEscalator
Showcasing Innovation Assay Genie recently took part in an engaging event at Oxfo …10th Jul 2025 -
Department of Oncology Retreat 2025
Department of Oncology RetreatAssay Genie was honoured to sponsor and participate in t …30th May 2025 -
Cure CLCN4 2025
Cure CLCN4 2025Assay Genie was honored to attend and present at the 2025 Cure CLCN4 Sc …26th May 2025




