The Rise of Alpha-Emitting Radiopharmaceuticals: A New Era in Targeted Cancer Therapy
Imagine a microscopic sniper, capable of delivering a lethal blow to a single cancer cell while leaving its healthy neighbors virtually untouched. This is the promise of targeted alpha therapy, a revolutionary frontier in nuclear medicine that is rapidly transforming the treatment landscape for advanced malignancies. By harnessing the immense energy of alpha particles, scientists are finally unlocking a way to overcome the resistance that has long plagued traditional cancer therapies, ushering in a new era of precision oncology.
Introduction
For decades, the field of radiopharmaceuticals was dominated by beta-emitting isotopes like Lutetium-177. While effective, beta particles are relatively low-energy and travel several millimeters through tissue, often causing 'collateral damage' to surrounding healthy cells. Enter alpha-emitting isotopes—such as Actinium-225 and Radium-223—which represent a paradigm shift in how we think about radiation. These particles are significantly heavier and carry much higher linear energy transfer (LET), meaning they deposit a massive amount of energy over a very short distance, typically only a few cell diameters. This high-density energy deposition causes irreparable double-strand breaks in DNA, making it nearly impossible for cancer cells to develop resistance. As clinical evidence mounts, it is becoming clear that alpha therapy represents a major oncology breakthrough, offering hope to patients with metastatic disease who have exhausted all other options. The transition from broad-spectrum radiation to these 'atomic bullets' marks one of the most significant advancements in the history of targeted cancer treatment.
The Heavy Hitters: Why Alpha Particles Outperform Traditional Isotopes
The biological superiority of alpha particles lies in their unique physical properties. Unlike beta particles, which might require hundreds of 'hits' to kill a cell, a single alpha particle traversal can be lethal. This is particularly crucial in treating 'cold' tumors or those with low oxygen levels, where traditional radiation often fails. Interestingly, the short range of alpha radiation—less than 100 micrometers—ensures that the toxic effects are confined almost entirely to the tumor mass. This precision allows for a much higher therapeutic index, meaning we can deliver more potent doses to the cancer while sparing the bone marrow and other sensitive organs. Beyond the physics, the clinical results are starting to speak for themselves. Recent multicenter studies have demonstrated that Actinium-225 shows high efficacy in advanced cancer, even in patients who have previously failed beta-emitter treatments like Lu-PSMA. This suggests that alpha therapy isn't just an alternative; it's a more powerful tool for the most difficult-to-treat cases.
The Double-Strand Break Advantage
The primary mechanism of cell death in alpha therapy is the induction of complex DNA damage that the cell's repair machinery simply cannot fix.
- High Linear Energy Transfer (LET) leads to dense ionization tracks.
- Direct DNA damage occurs independently of oxygen concentration (overcoming hypoxia).
- Minimal risk of secondary cancers due to the extremely short range of the particles.
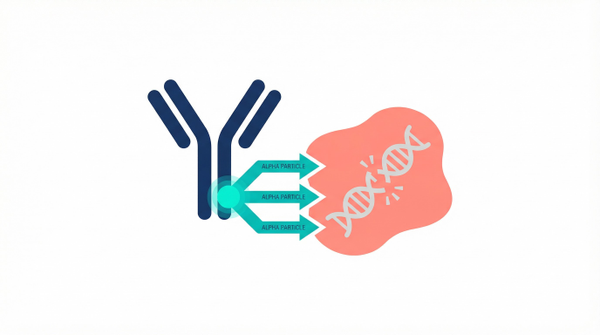
Precision Delivery: How Antibodies and Ligands Find Their Mark
An 'atomic bullet' is only as good as its guidance system. To ensure alpha emitters reach the tumor, they must be attached to targeting molecules—usually monoclonal antibodies or small-molecule ligands—that seek out specific proteins on the surface of cancer cells. One of the most successful targets has been the Prostate-Specific Membrane Antigen (PSMA). However, the challenge has always been ensuring the isotope stays attached to the carrier until it reaches the target. Recent innovations in chemical engineering are solving this. For instance, researchers have found that covalent binding enhances targeted alpha particle delivery, creating a more stable and potent therapeutic complex. This stability is vital because it prevents the 'leakage' of radioactive isotopes into the bloodstream, which could otherwise cause off-target toxicity. Furthermore, the choice of carrier can drastically change the drug's behavior in the body. While small ligands clear the system quickly, antibodies provide a longer circulation time, which can be advantageous for certain tumor types. A landmark phase I study recently confirmed that antibody-delivered actinium-225 is safe and effective, paving the way for more sophisticated delivery platforms across a variety of solid tumors.
The PSMA Revolution in Prostate Cancer
Prostate cancer has become the 'proving ground' for alpha therapy due to the high expression of PSMA in metastatic lesions.
- J591 antibodies allow for precise targeting of the PSMA extracellular domain.
- Small molecule ligands offer rapid tumor penetration and clearance.
- New chelators are being developed to hold Actinium-225 more securely.
The Power of Two: Synergistic Combinations and the 'AlphaBet' Strategy
While alpha therapy is potent on its own, the future likely lies in combination treatments. Doctors are now exploring the 'AlphaBet' strategy—combining alpha emitters with beta emitters to cover all bases. The logic is sound: beta particles handle the larger tumor masses with their longer range, while alpha particles 'mop up' the microscopic clusters and resistant cells. This dual-action approach is showing remarkable promise in early trials. Specifically, an interim analysis has shown that combining alpha and beta emitters improves outcomes compared to using either alone. But the synergy doesn't stop there. Alpha radiopharmaceuticals are also being paired with external beam radiation to sensitize tumors. In the RAVENS trial, researchers observed that Radium-223 combined with radiotherapy extends survival in patients with oligometastatic disease. This suggests that alpha therapy can act as a force multiplier, enhancing the effectiveness of existing standard-of-care treatments and offering a multi-pronged assault on the cancer's defenses.
Overcoming Tumor Heterogeneity
Tumors are rarely uniform; they are mosaics of different cell types with varying levels of target expression.
- Beta particles provide a 'crossfire effect' to kill cells that don't express the target protein.
- Alpha particles ensure the death of the most aggressive, radiation-resistant clones.
- Combination therapy reduces the likelihood of the tumor 'escaping' treatment through mutation.
Beyond Prostate Cancer: Expanding the Alpha Frontier
While much of the current excitement centers on prostate cancer, the potential for alpha therapy extends far beyond a single disease. Researchers are already looking at targeting alpha emitters to neuroendocrine tumors, glioblastoma, and even certain types of leukemia. The beauty of the technology is its modularity: if you can find a unique protein on a cancer cell, you can theoretically target it with an alpha emitter. However, expanding this frontier requires overcoming significant logistical hurdles. The production of isotopes like Actinium-225 is currently limited, and the specialized facilities required for handling these materials are few and far between. Despite these challenges, the momentum is undeniable. As we refine our targeting strategies and scale up production, alpha-emitting radiopharmaceuticals are poised to move from 'last-resort' treatments to first-line therapies. We are witnessing the birth of a new pillar in oncology, one that combines the raw power of nuclear physics with the delicate precision of molecular biology to give patients a fighting chance they never had before.
A New Dawn for Targeted Therapy
The rise of alpha-emitting radiopharmaceuticals represents more than just a new drug class; it is a fundamental shift in our ability to fight metastatic cancer. By delivering high-energy radiation with sub-cellular precision, we are finally beginning to close the gap between treatment and cure for many patients. While challenges in isotope supply and clinical implementation remain, the data from high-impact journals like Nature and Lancet Oncology confirm that we are on the right path. As we continue to refine these 'atomic snipers,' the dream of a truly personalized, highly effective, and low-toxicity cancer treatment is becoming a reality. The era of alpha therapy has arrived, and for millions of patients worldwide, the future has never looked brighter.
References
- Kostos L, Buteau JP, Xie J, et al. (2025). Lutetium-177 [(177)Lu]Lu-PSMA-I&T plus radium-223 in patients with metastatic castration-resistant prostate cancer (AlphaBet): an interim analysis of the investigator-initiated, single-centre, single-arm, phase 1/2 trial. Lancet Oncol. 26(11):1479-1488. PMID: 41119954
- Sathekge MM, Lawal IO, Bal C, et al. (2024). Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 25(2):175-183. PMID: 38218192
- Tagawa ST, Thomas C, Sartor AO, et al. (2024). Prostate-Specific Membrane Antigen-Targeting Alpha Emitter via Antibody Delivery for Metastatic Castration-Resistant Prostate Cancer: A Phase I Dose-Escalation Study of 225Ac-J591. J Clin Oncol. 42(7):842-851. PMID: 37922438
- Cui XY, Li Z, Kong Z, et al. (2024). Covalent targeted radioligands potentiate radionuclide therapy. Nature. 630(8015):206-213. PMID: 38778111
- Wang JH, Sherry AD, Bazyar S, et al. (2025). Outcomes of Radium-223 and Stereotactic Ablative Radiotherapy Versus Stereotactic Ablative Radiotherapy for Oligometastatic Prostate Cancers: The RAVENS Phase II Randomized Trial. J Clin Oncol. 43(18):2059-2068. PMID: 40334149
- Kairemo K. (2024). Targeted alpha therapy: a new tool for advanced prostate cancer. Lancet Oncol. 25(2):148-149. PMID: 38218191
Recent Posts
-
The Rise of Alpha-Emitting Radiopharmaceuticals: A New Era in Targeted Cancer Therapy
Imagine a microscopic sniper, capable of delivering a lethal blow to a single cancer cell while lea …19th Feb 2026 -
Targeted Protein Degradation: The Next Frontier in Oncology Drug Discovery
For decades, cancer drug development has focused on blocking proteins—inhibiting enzymes, antagoniz …18th Feb 2026 -
Neuroinflammation and Compulsive Behavior: The Role of Astrocytes in OCD
Imagine a mind trapped in a loop, endlessly repeating actions or thoughts, driven by an invisible f …18th Feb 2026