Trauma Immunology
Traumatic injuries & death
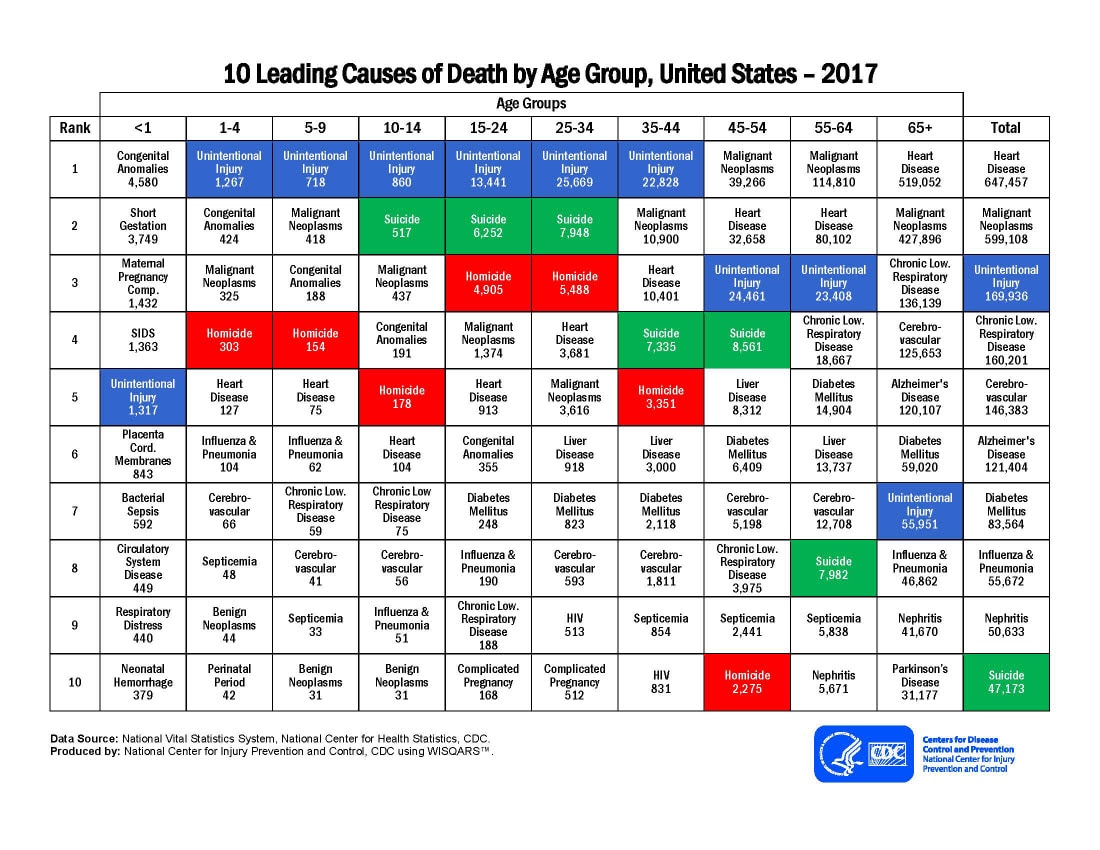
When I ask people what the leading cause of death is in the United States in people age 44 and under, they generally reply saying cancer. It wouldn’t be illogical to think that this is the answer, but it would be incorrect. In fact, unintentional traumatic injuries are responsible for more deaths in these age groups than any other cause such as homicide, cancer or heart disease. Accidental traumatic injury can be categorized into several groups such as blunt-force trauma, hemorrhage, crush injuries, bone fractures, radiation injury and any of these in combination. These types of injuries happen in traumatic scenarios like motor vehicle accidents, fires, shootings, stabbings, accidental falls and so on.
Clinical management of trauma
The clinical management of trauma has improved significantly over the past several decades, however, further advancements are somewhat impeded when trauma patients develop post-injury bacterial infections that can lead to sepsis. It has been known for some time that the immune system is altered by trauma, but the complex nature of traumatic injuries has made it difficult to perform comprehensive and extensive analyses of the multiple immune cell types affected by injury.
Immunological response to trauma
When a person is injured, the immune system responds by reacting to tissue damage and “danger molecules” or “alarmins” that activate immune cells and ultimately disrupt immune cell homeostasis. This imbalance across the entire immune system subsequently predisposes the patient to opportunistic infections and inflammatory complications. Studies addressing the effects of trauma on the immune system point to a dynamic physiological reaction. This response evolved to initiate pro-inflammatory activity in response to cell and tissue damage that releases previously sequestered antigens. However, the counter response of the immune system is to then reduce this damage reactive, trauma-induced inflammation by mounting an anti-inflammatory cascade of events.
Unfortunately, these opposing reactions occur at the same time and can lead to the suppression of antimicrobial immunity resulting in susceptibility to opportunistic infection and mortality at timepoints when the imbalance is greatest, typically around day 7 after injury (Fig. 1). Therefore, treatments should be aimed at supporting the control of immune reactivity and the concurrent promotion of an immune response capable of resisting nosocomial infections.
As such, my role as a Postdoctoral Research Fellow in the Trauma Immunology Laboratory (http://ledererlab.bwh.harvard.edu) at Brigham and Women’s Hospital/Harvard Medical School is to better understand the changes in the immune system that occur because of traumatic injuries and contribute to loss of immune function. Ultimately, findings from my research will contribute to the discovery of cellular and molecular targets to reduce post-injury nosocomial infections and trauma-induced inflammatory complications.
Systems immunology approach to understand trauma
I am currently using systems immunology research approaches to advance our understanding of the immunological consequences of trauma to provide novel insights into how traumatic injuries affect the immune system phenotypes and functions. A central part of my research uses CyTOF (cytometry by time-of-flight) mass cytometry, an innovative technology that allows for comprehensive single-cell immune profiling. This technology relies on rare-earth metal tagged antibodies and time-of-flight mass spectrometry for high resolution cell stains.
CyTOF is similar to flow cytometry but because it uses rare-earth metal isotope labeled antibodies to detect cell markers, it allows for multiplex staining with minimal signal overlap over a wide mass range (Fig. 2). Thus, simultaneous staining of cells with reagents specific for up to 40 different cell markers in a single sample can be achieved by CyTOF. In addition, because CyTOF detection uses rare-earth element isotopes that are not normally found in cells there is minimal background signal and low signal overlap which can be problematic in flow cytometry.
Activation of Tregs by traumatic injury
Previous research from the Trauma Immunology laboratory identified that a specific type of T cell called Regulatory T cells (Tregs) were activated by traumatic injury. Tregs are a subset of thymic derived CD4+ T cells, which have been shown to be central to the maintenance of immunological tolerance and controlling inflammation in autoimmune and inflammatory disease mouse models. Furthermore, they develop T cell receptor repertoires that are reactive to endogenous antigens. This self-reactive capacity of some Tregs may support a role for these cells specifically reacting to tissue injury as a result of trauma. These complex biological features of Tregs suggest that they are distinctively suited to controlling immune responses. It has not yet been addressed in detail if Tregs are beneficial or disadvantageous in trauma and thus I am interested in discovering how Tregs are activated by trauma and their role in modulating trauma-induced immune reactions. Elucidating their role in trauma immunity through basic research may provide new insights into why some injured patients fare better than others, generating data that will help the field better understand how to restore immune homeostasis in trauma patients and prevent the development of life-threatening infections.
Future traumatic injury research
Surviving the initial injury, whether it is a broken leg from a fall or severe bleeding and organ damage from a gunshot wound, is just the first hurdle. We hear on the news of people surviving devastating mass shootings or motor vehicle accidents but more often than not, that is just the foundational injury. We don’t hear of how well they are doing in hospital afterwards or if they developed opportunistic infections and succumbed to post-injury sepsis. Incredible strides have been made in furthering our understanding of how trauma influences the immune system through basic research and clinical studies but traumatic accidents remain the leading cause of death in the United States in age groups up to 44 years old (and remains a major cause of death in age groups 44 and upwards). This highlights the need for further advancement of our current knowledge of the immunological reaction to traumatic injury. I am fortunate and inspired to be in the position to discover novel insights into how cells of the immune system are activated and modulated by trauma.
1) https://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_by_age_group_2017_1100w850h.jpg
2) Akinori Osuka et. al., (2014) Immune response to traumatic injury: harmony and discordance of immune system homeostasis. Acute Medicine & Surgery, Apr; 1(2): 63–69
3) Bandura, D.R. et al., Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009)
4) Sakaguchi S., et.al., (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27
5) Miyara M., Sakaguchi S. (2007) Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 13, 108–116
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026