CD80: Amplifying Immune Activation Through Co-Stimulatory Pathways
Introduction to CD80 and Its Role in Immune Activation
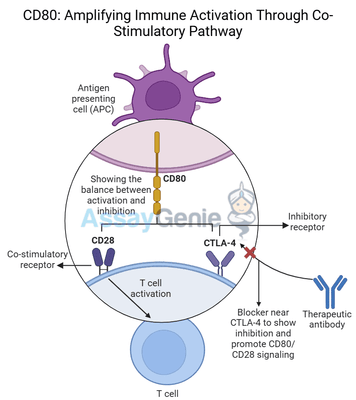
CD80 is a critical immune checkpoint molecule that plays an essential role in T cell activation through co-stimulatory pathways. Expressed primarily on antigen-presenting cells (APCs) such as dendritic cells, B cells, and macrophages, CD80 interacts with receptors on T cells to regulate immune responses. Specifically, CD80 engages CD28 to provide a crucial co-stimulatory signal necessary for full T cell activation, proliferation, and differentiation. This co-stimulation amplifies the immune system's ability to recognize and attack pathogens, as well as cancer cells.
In addition to its role in immune activation, CD80 can also bind to CTLA-4, an inhibitory receptor on T cells, which dampens immune responses and promotes immune tolerance. This dual functionality of CD80 in both activating and inhibiting immune responses makes it an important target in cancer immunotherapy, where modulating the balance between immune activation and suppression is key to enhancing anti-tumor immunity.
Monoclonal antibodies such as 16-10A1, which target CD80, have emerged as potential tools for manipulating immune responses in cancer therapy. This article delves into the biology of CD80, its role in T cell co-stimulation, and the therapeutic potential of CD80-targeting agents in amplifying immune responses against cancer.
The Structure and Function of CD80 in T Cell Co-Stimulation
CD80 and Its Interaction with CD28
CD80 belongs to the B7 family of immune checkpoint molecules, which play pivotal roles in regulating immune responses by delivering either co-stimulatory or inhibitory signals to T cells. When an antigen-presenting cell (APC) presents an antigen to a T cell, the engagement of the T cell receptor (TCR) with the antigen-MHC complex provides the primary signal for T cell activation. However, this signal alone is not sufficient for full activation. T cells require a second signal—known as co-stimulation—to become fully activated, proliferate, and differentiate into effector T cells capable of targeting pathogens or cancer cells.
CD80 provides this second signal by binding to CD28, a receptor on the surface of T cells. The CD80-CD28 interaction delivers a potent co-stimulatory signal that promotes:
- T cell proliferation: CD28 signaling enhances the expansion of activated T cells.
- Cytokine production: Co-stimulation by CD80 increases the production of key cytokines like IL-2, which further supports T cell growth and differentiation.
- Enhanced T cell survival: CD80 engagement promotes the long-term survival of activated T cells, ensuring a sustained immune response.
By amplifying these processes, CD80 plays a central role in shaping robust anti-tumor immune responses.
CD80 and Its Interaction with CTLA-4
While CD80 provides co-stimulatory signals through CD28, it can also bind to CTLA-4, a checkpoint receptor expressed on activated T cells and regulatory T cells (Tregs). Unlike CD28, CTLA-4 delivers inhibitory signals that suppress T cell activity, acting as a natural brake to prevent excessive immune responses that could lead to tissue damage or autoimmunity.
When CD80 engages CTLA-4:
- T cell activity is suppressed: CTLA-4 signaling reduces T cell proliferation and cytokine production.
- Regulatory T cells (Tregs) are promoted: CTLA-4 enhances the activity of Tregs, which play a
key role in maintaining immune tolerance and preventing autoimmunity.
This dual interaction with both CD28 and CTLA-4 enables CD80 to balance immune activation and suppression, making it a complex but valuable target for cancer immunotherapy.
CD80 in the Tumor Microenvironment
CD80 and Immune Evasion in Cancer
In the tumor microenvironment (TME), cancer cells and tumor-associated immune cells often exploit immune checkpoints like CD80 to suppress immune responses and evade destruction by the immune system. Tumor cells may increase the expression of CTLA-4 ligands such as CD80 to inhibit T cell activation and promote immune evasion. By engaging CTLA-4 on T cells, CD80 helps create an immunosuppressive microenvironment that favors tumor growth and survival.
High levels of CD80 expression on tumor cells or tumor-associated macrophages (TAMs) are associated with poor prognosis in several cancer types, including:
In these tumors, CD80-mediated suppression of T cells allows cancer cells to evade immune detection and resist immune-mediated killing.
Therapeutic Potential of Targeting CD80 in Cancer
Targeting CD80 in cancer therapy aims to block its inhibitory signals through CTLA-4, thereby restoring T cell activity and enhancing the immune system's ability to fight cancer. Monoclonal antibodies like 16-10A1 have been developed to disrupt the interaction between CD80 and CTLA-4, tipping the balance in favor of immune activation by allowing CD80 to provide co-stimulatory signals through CD28.
By preventing CD80 from binding to CTLA-4, CD80-targeting therapies can:
- Reinvigorate T cell responses: Blocking CD80-CTLA-4 interactions enhances T cell proliferation, cytokine production, and cytotoxic activity, promoting a stronger anti-tumor response.
- Reduce regulatory T cell activity: Inhibiting CD80-CTLA-4 binding decreases Treg-mediated immunosuppression, allowing effector T cells to function more effectively.
- Enhance immune checkpoint blockade: CD80-targeting therapies can synergize with checkpoint inhibitors like anti-PD-1 and anti-CTLA-4, providing a more comprehensive approach to immune modulation in cancer treatment.
16-10A1: A Monoclonal Antibody Targeting CD80
Mechanism of Action of 16-10A1
16-10A1 is a monoclonal antibody designed to specifically block CD80 from binding to CTLA-4, thereby enhancing T cell activation and immune responses against cancer. The mechanism of action of 16-10A1 involves:
- Blocking CD80-CTLA-4 interaction: 16-10A1 binds to CD80, preventing it from engaging CTLA-4 and delivering inhibitory signals to T cells.
- Enhancing T cell-mediated cytotoxicity: By blocking the inhibitory pathway, CD80 is more available to bind to CD28, amplifying T cell activation and proliferation.
- Enhancing T cell-mediated cytotoxicity: Increased CD28 signaling boosts the ability of T cells to produce cytokines, proliferate, and attack tumor cells.
- Modulating the tumor microenvironment: 16-10A1 reduces the suppressive effects of Tregs and tumor-associated macrophages, shifting the TME towards a more immune-activating
state.
Clinical Applications of 16-10A1
The therapeutic potential of 16-10A1 is being explored in various cancers, particularly those with high levels of immune suppression driven by CD80-CTLA-4 interactions. These include:
- Melanoma: CD80-CTLA-4 signaling is a key mechanism of immune evasion in melanoma, and blocking this interaction with 16-10A1 can enhance T cell responses against the tumor.
- Breast Cancer: Tumors that express high levels of CD80 often promote immune tolerance,
making CD80-targeting antibodies like 16-10A1 a promising approach to reinvigorating immune activity. - Non-Small Cell Lung Cancer (NSCLC): The immunosuppressive TME in NSCLC can be modulated by targeting CD80, improving the efficacy of other immunotherapies.
Cancer Type | CD80 Expression | Potential of CD80 Blockade (16-10A1) |
|---|---|---|
High on tumor-associated immune cells | Blocking CD80-CTLA-4 enhances T cell activity and immune response. | |
Elevated in immunosuppressive TME | CD80 blockade reduces immune suppression and promotes anti-tumor immunity. | |
Upregulated in the tumor microenvironment | 16-10A1 improves T cell function and enhances responses to checkpoint inhibitors. |
Synergy with Checkpoint Inhibitors and Other Therapies
Combination with Anti-PD-1 and Anti-CTLA-4 Therapies
Checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 have shown remarkable success in treating various cancers by releasing the immune system’s brakes. However, some tumors remain resistant to these therapies due to multiple immune-suppressive pathways. Targeting CD80 with 16-10A1 can complement these treatments by:
- Enhancing T cell activation: CD80-targeting therapies boost co-stimulatory signals, making T cells more responsive to checkpoint inhibitors.
- Overcoming resistance: In tumors where checkpoint inhibitors alone are insufficient, blocking
CD80-CTLA-4 interactions can provide an additional mechanism to overcome immune suppression and improve treatment outcomes.
Potential for Combining CD80 Blockade with Chemotherapy
Chemotherapy can cause the release of tumor antigens, which are recognized by the immune system. However, chemotherapy-induced immunosuppression often limits the effectiveness of these responses. By targeting CD80, therapies like 16-10A1 can enhance the immune system’s ability to respond to these antigens, making chemotherapy more effective.
Challenges and Future Directions in CD80-Targeted Therapy
Managing Immune-Related Toxicities
Like other immune-modulating therapies, blocking CD80-CTLA-4 interactions can lead to immune-related adverse events (irAEs), including inflammation and autoimmunity. Careful dosing and patient monitoring will be essential to minimize these risks while maximizing the therapeutic benefits of CD80-targeting antibodies.
Expanding the Use of CD80 Blockade
Research into CD80-targeting therapies is still in its early stages, but future studies will likely explore their application in a broader range of cancers. Identifying biomarkers that predict patient response to CD80-targeted therapies will be critical for expanding their use and ensuring the most appropriate patient selection.
Conclusion
CD80 is a powerful immune checkpoint molecule that plays a central role in T cell co-stimulation and immune regulation. By targeting CD80 with therapies like 16-10A1, cancer immunotherapy can tip the balance toward immune activation, amplifying T cell responses and overcoming tumor-induced immune suppression. As research into CD80-targeted therapies progresses, these approaches hold great promise for enhancing the efficacy of existing treatments and improving outcomes for patients with immune-resistant cancers.
References
- Linsley, P.S., et al., 1991. Identification of a novel T cell co-stimulatory receptor CD28 and its ligands, CD80 (B7) and CD86. The Journal of Experimental Medicine, 174(3), pp.561-569.
- Chambers, C.A., Kuhns, M.S., Egen, J.G., et al., 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunology, 19(1), pp.565-594.
- Quezada, S.A., Peggs, K.S., Simpson, T.R., et al., 2011. CTLA4 blockade and Treg depletion cooperate to enhance T cell responses in vivo. The Journal of Experimental Medicine, 208(7),
pp.1491-1503. - Chambers, C.A., et al., 1999. The CD28-B7 pathway inthe regulation of T-cell responses. Immunological Reviews, 169(1), pp.55-66.
- Bour-Jordan, H., et al., 2011. The relationship between CD28 and CTLA-4 in the regulation of T-cell responses. Advances in Immunology, 109(1), pp.1-26.
- Mahoney, K.M., Freeman, G.J., 2015. The immunoregulatory role of B7 family members in antitumor immunity. Nature Reviews Immunology, 15(1), pp.72-82.
- Schneider, H., et al., 2002. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. Journal of Immunology, 169(6), pp.3048-3054.
Recent Posts
-
Department of Oncology Retreat 2025
Department of Oncology RetreatAssay Genie was honoured to sponsor and participate in t …30th May 2025 -
Cure CLCN4 2025
Cure CLCN4 2025Assay Genie was honored to attend and present at the 2025 Cure CLCN4 Sc …26th May 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …26th May 2025