Elotuzumab: Revolutionizing Multiple Myeloma Treatment and Research Applications
Quick Facts About Elotuzumab
What is Elotuzumab?
Elotuzumab is a monoclonal antibody designed to enhance the immune system's ability to detect and destroy multiple myeloma cells. It targets the SLAMF7 protein, which is present on both myeloma and immune natural killer (NK) cells.
How does Elotuzumab work?
Elotuzumab activates NK cells by binding to SLAMF7, boosting their ability to attack cancer cells. It also directly marks myeloma cells for immune system destruction, making it a dual-action therapy.
What is Elotuzumab used for?
Primarily, Elotuzumab is used in combination therapies for relapsed or refractory multiple myeloma. These include pairings with drugs like lenalidomide, pomalidomide, and dexamethasone.
1.) Understanding Elotuzumab
Elotuzumab represents a significant advancement in immunotherapy, particularly for treating multiple myeloma. It selectively binds to SLAMF7, a protein highly expressed on myeloma cells and natural killer (NK) cells, enhancing immune-mediated destruction of cancer cells. Since its FDA approval, Elotuzumab has been a cornerstone in combination therapies for relapsed or refractory multiple myeloma.
Prefer to Listen? Check out the Elotuzumab Podcast Episode
2.) Mechanism of Action of Elotuzumab
Elotuzumab operates through dual mechanisms:
2.) Direct Targeting: By attaching to SLAMF7 on myeloma cells, it flags them for destruction by the immune system.
This dual mechanism makes Elotuzumab a vital therapeutic option, particularly when used in combination with immunomodulatory drugs.

3.) Clinical Applications of Elotuzumab
Elotuzumab has proven efficacy in multiple myeloma treatment regimens. Common combinations include
- Elotuzumab with Lenalidomide and Dexamethasone: Approved for newly diagnosed and relapsed cases.
- Elotuzumab with Pomalidomide and Dexamethasone: Typically used for patients with relapsed or refractory multiple myeloma.
Ongoing research explores new combinations and its potential in other hematologic malignancies.
4.) Advancing Research on Elotuzumab with Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an existing FDA-approved biologic, known as the reference product. Biosimilars offer comparable safety, purity, and potency while being more cost-effective, enhancing research accessibility.
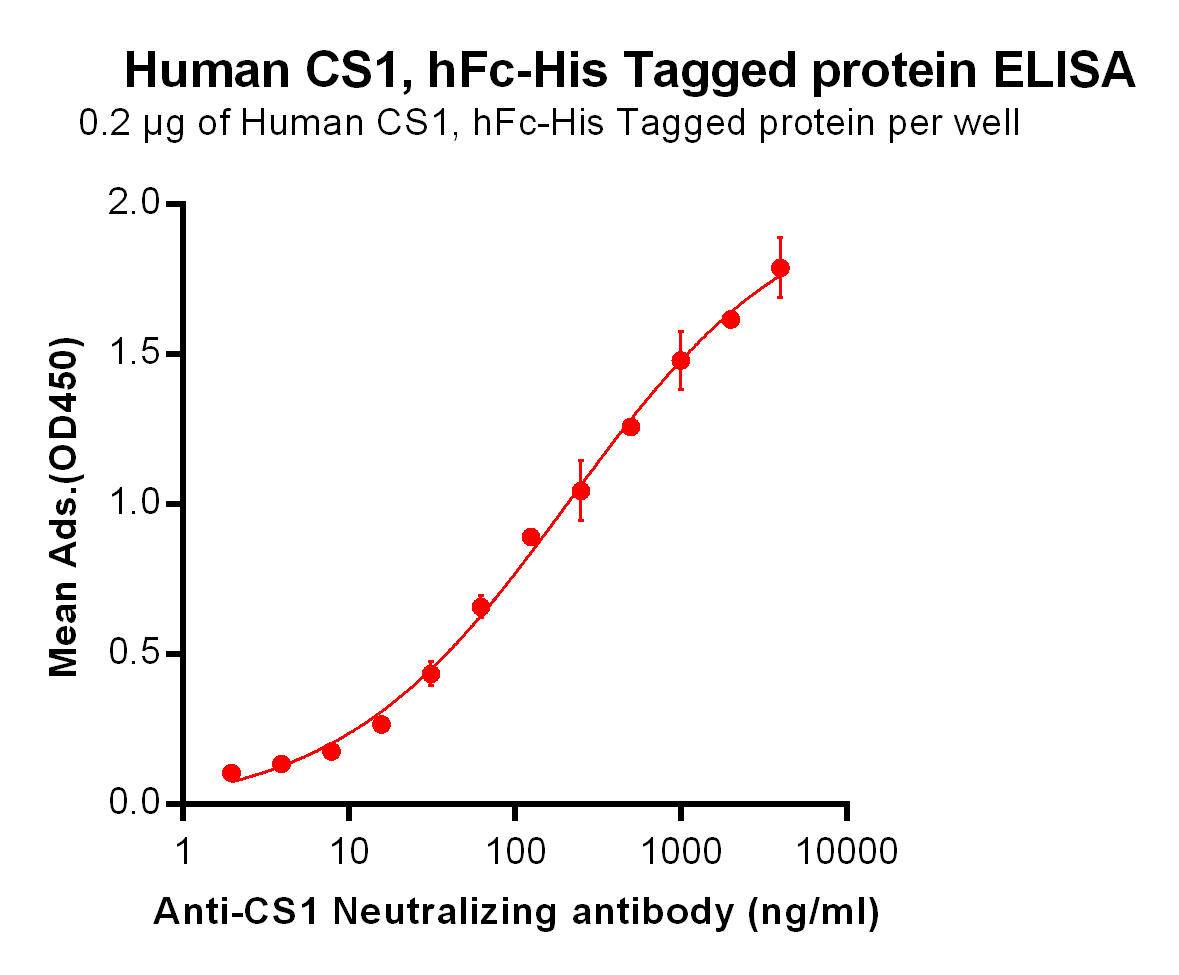
| Elotuzumab (Anti-CS1) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CS1 |
| Reactivity: | Human |
The Role of Elotuzumab Biosimilars in Research
- SLAMF7 targeting mechanisms.
- Combination therapies in preclinical settings.
- Pathways involved in immune-mediated cancer cell destruction.
Benefits of Elotuzumab Biosimilars
- Cost-Effective : Reduces research expenses compared to reference products.
- High Quality: Maintains rigorous standards for research applications.
- Research-Use Only: Ideal for experimental and preclinical studies, ensuring compliance with regulatory guidelines.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Exhibition at the University of Oxford's BioEscalator
Showcasing Innovation Assay Genie recently took part in an engaging event at Oxfo …10th Jul 2025 -
Department of Oncology Retreat 2025
Department of Oncology RetreatAssay Genie was honoured to sponsor and participate in t …30th May 2025 -
Cure CLCN4 2025
Cure CLCN4 2025Assay Genie was honored to attend and present at the 2025 Cure CLCN4 Sc …26th May 2025




