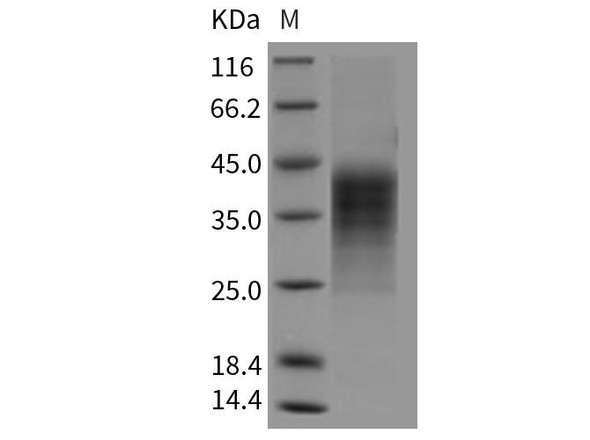
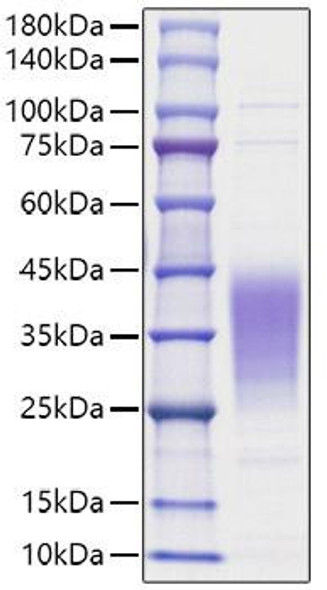
Description
Recombinant Human LIF Protein (KPRT0313)
| Product SKU | KPRT0313 |
| Product Type | Recombinant Protein |
| Quantity | 5 ug |
| Species | Human |
| Target | LIF |
| Alias | LIF; CDF; DIA; HILDA; MLPLI |
| Source | Yeast |
| No. Amino Acids | 180 |
| Protein Sequence | SPLPITPVNA TCAIRHPCHN NLMNQIRSQL AQLNGSANAL FILYYTAQGE PFPNNLDKLC GPNVTDFPPF HANGTEKAKL VELYRIVVYL GTSLGNITRD QKILNPSALS LHSKLNATAD ILRGLLSNVL CRLCSKYHVG HVDVTYGPDT SGKDVFQKKK LGCQLLGKYK QIIAVLAQAF (180) |
| MW | 19.7 kDa |
| Form | Lyophilized |
| Storage | -20°C |
| Shipping Conditions | Room temperature |