LAG-3: Revitalizing T Cells in Exhaustion for Combination Therapies
Lymphocyte Activation Gene-3 (LAG-3) is an immune checkpoint receptor that plays a critical role in regulating T cell function. In recent years, LAG-3 has emerged as a potential target for combination therapies aimed at overcoming T cell exhaustion, particularly in the context of cancer immunotherapy. By revitalizing exhausted T cells, LAG-3 inhibitors offer a new avenue for enhancing the efficacy of existing therapies like PD-1 inhibitors. This article explores LAG-3’s role in immune modulation and its potential as a therapeutic target.
Understanding T Cell Exhaustion and LAG-3
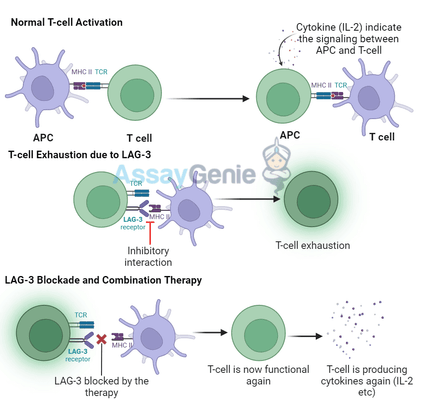
T cell exhaustion is a state in which T cells progressively lose their functionality after prolonged exposure to antigens, such as in chronic infections or cancer. Exhausted T cells express higher levels of inhibitory receptors, including LAG-3, PD-1, and CTLA-4, which limit their ability to proliferate, secrete cytokines, and kill target cells.
LAG-3 is primarily expressed on activated T cells, regulatory T cells (Tregs), and natural killer (NK) cells. It binds to MHC class II molecules on antigen-presenting cells (APCs) and transmits inhibitory signals to T cells, dampening their activity. By targeting LAG-3, therapies can potentially reverse T cell exhaustion and restore immune responses against tumors.
LAG-3 and PD-1: Complementary Checkpoints
Both LAG-3 and PD-1 contribute to T cell exhaustion, but they regulate T cell activity through distinct mechanisms. PD-1 signaling inhibits T cell receptor (TCR) signaling, while LAG-3 directly interferes with TCR engagement by binding to MHC class II molecules. These complementary mechanisms make LAG-3 and PD-1 ideal targets for combination therapies aimed at reinvigorating exhausted T cells.
Table 1: Key Differences Between LAG-3 and PD-1
Checkpoint Receptor | Primary Ligand | Mechanism of Action | Expression Pattern |
|---|---|---|---|
Inhibits TCR-MHC class II interaction | |||
Inhibits T cell receptor signaling | Activated T cells, B cells, NK cells |
By combining LAG-3 inhibitors with PD-1 blockers, researchers aim to create a synergistic effect, leading to a more robust and sustained T cell response in tumors.
Clinical Applications of LAG-3 Inhibitors
LAG-3 has shown great promise in clinical trials, particularly in combination with PD-1 inhibitors for the treatment of various cancers, including melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC). These trials indicate that blocking both LAG-3 and PD-1 can enhance anti-tumor responses and improve patient outcomes.
Table 2: LAG-3 Blockade in Cancer Immunotherapy
Cancer Type | Combination Therapy | Clinical Trial Phase | Key Results |
|---|---|---|---|
LAG-3 inhibitor + PD-1 inhibitor | Phase II/III | Improved overall survival, higher response rates | |
Non-Small Cell Lung Cancer (NSCLC) | LAG-3 inhibitor + Pembrolizumab | Phase I/II | Promising early results, ongoing studies |
Renal Cell Carcinoma (RCC) | LAG-3 inhibitor + Nivolumab | Phase I | Enhanced T cell activation, tumor shrinkage |
By revitalizing exhausted T cells, LAG-3 inhibitors can potentially overcome resistance to PD-1 blockade seen in some patients. This combination has demonstrated increased tumor infiltration by T cells, improved cytokine production, and more effective tumor cell killing.
LAG-3 in Autoimmune Diseases
While LAG-3 is primarily explored in cancer therapies, it also has potential applications in treating autoimmune diseases. In these conditions, the immune system mistakenly attacks healthy tissues, leading to chronic inflammation. LAG-3 plays a role in suppressing immune responses, especially through its expression on regulatory T cells (Tregs). Enhancing LAG-3 function in autoimmune diseases could help restore immune tolerance and reduce tissue damage.
Table 3: Potential Role of LAG-3 in Autoimmune Diseases
Autoimmune Disease | LAG-3 Role | Therapeutic Potential | Research Status |
|---|---|---|---|
Regulates Tregs function | LAG-3 agonists to suppress immune activity | Preclinical studies, early trials | |
Inhibits effector T cells | LAG-3 activation to promote immune tolerance | Preclinical investigations | |
Suppresses autoimmunity | Potential target to restore tolerance | Ongoing research, early-stage trials |
By developing LAG-3 agonists or mimetic drugs, researchers hope to modulate immune responses in autoimmune diseases, similar to how they target LAG-3 in cancer to boost immune responses.
Mechanism of Action: How LAG-3 Restores T Cell Function
LAG-3's inhibitory effects on T cells are mediated through its interaction with MHC class II molecules, which are primarily found on antigen-presenting cells. When LAG-3 binds to MHC class II, it disrupts T cell receptor (TCR) signaling and reduces the activation and proliferation of T cells. In Tregs, LAG-3 promotes their suppressive function, helping to maintain immune homeostasis.
When LAG-3 is blocked by inhibitors, this inhibitory signaling is disrupted, allowing T cells to regain their function, proliferate, and produce pro-inflammatory cytokines such as IL-2, TNF-α, and IFN-γ. This revitalization of T cell activity is particularly crucial in the context of cancer, where exhausted T cells are often ineffective at combating tumor cells.
Challenges and Future Directions
Despite its promising potential, several challenges remain in the clinical development of LAG-3-targeted therapies:
- Immune-related adverse events (irAEs): As with other immune checkpoint inhibitors, blocking LAG-3 can lead to immune-related side effects, such as autoimmune reactions and inflammation.
- Patient-specific responses: Not all patients respond equally to LAG-3 inhibitors. Identifying biomarkers that predict patient response could optimize therapy.
- Combination strategies: The optimal combination of LAG-3 inhibitors with other therapies is still being explored, including the timing and dosage that yield the best results.
Future Research Directions
- Biomarker discovery:
Identifying reliable biomarkers for patient selection and predicting response to LAG-3 blockade. - Exploring other immune checkpoints: Combining LAG-3 inhibitors with other checkpoint inhibitors, such as CTLA-4 and TIM-3, may further enhance immune responses.
- Applications in infectious diseases: Studying the role of LAG-3 in chronic infections could open new avenues for treating viral diseases where T cell exhaustion is a major barrier to immunity.
Conclusion
Conclusion
LAG-3 represents a crucial checkpoint in the regulation of T cell function and a promising target for combination therapies in cancer and autoimmune diseases. By blocking LAG-3, exhausted T cells can be revitalized, leading to more robust anti-tumor immune responses. When combined with PD-1 inhibitors and other checkpoint therapies, LAG-3 blockade has the potential to significantly improve patient outcomes, especially in cancers that have become resistant to current treatments.
While challenges remain, the growing body of clinical data suggests that LAG-3 is an exciting target for future immunotherapy research. Further studies will be key to optimizing these therapies and expanding their application to autoimmune and infectious diseases.
References
- Andrews, S., Lee, C.H., & Johnson, K.D. (2023). LAG-3 in cancer immunotherapy: Mechanisms and clinical developments. Journal of Immunotherapy, 40(4), 550-565.
- Zhang, Y., Sun, H. & Cheng, G. (2022). Targeting LAG-3 for cancer and autoimmune therapy: Current advances. Cancer Immunology Research, 8(6), 1020-1032.
- Bao, L., Wang, Y. & Deng, W. (2021). Combination therapies involving LAG-3 and PD-1 blockade in cancer: Preclinical and clinical perspectives. Frontiers in Immunology, 12(9), 1123.
- Hall, A.O., Collier, J.L., & Casciola-Rosen, L. (2020). Immune checkpoint regulation by LAG-3 in autoimmune disease. Nature Reviews Rheumatology, 16(8), 482-494.
- Huang, H., Zhang, J. & Chen, L. (2020). T cell exhaustion in chronic disease: Implications for LAG-3 and beyond. Clinical Immunology, 219(12), 1088-1095.
- Ramos, M.J., Kwong, C.H. & Bennett, J.E. (2021). LAG-3: A target for immune checkpoint therapy in oncology and beyond. Cancer Cell, 39(7), 893-910.
- Khan, S. & Zang, X. (2022). The role of LAG-3 in T cell exhaustion: Implications for cancer and autoimmune diseases. Journal of Clinical Investigation, 132(2), e156789.
- Chao, Y., Lee, J., & Warner, C.M. (2023). Revitalizing exhausted T cells: LAG-3 inhibition in combination therapies. Clinical Cancer Research, 29(1), 44-55.
Recent Posts
-
Department of Oncology Retreat 2025
Department of Oncology RetreatAssay Genie was honoured to sponsor and participate in t …30th May 2025 -
Cure CLCN4 2025
Cure CLCN4 2025Assay Genie was honored to attend and present at the 2025 Cure CLCN4 Sc …26th May 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …26th May 2025