Description
Recombinant Human Lipocalin-2/NGAL/LCN2 Protein
The Recombinant Human Lipocalin-2/NGAL/LCN2 Protein is a biologically active recombinant protein that plays a significant role in various cellular processes and signaling pathways in human biology. This protein is widely employed in immunological research, cell biology studies, protein-protein interaction analyses, and therapeutic development, providing researchers with a reliable tool for investigating Lipocalin-2/NGAL/LCN2 function and its implications in health and disease.
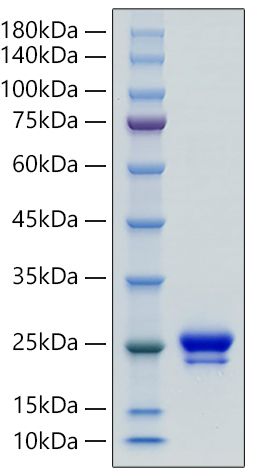
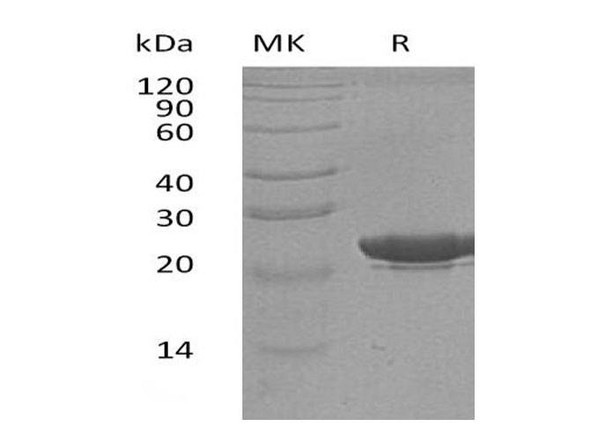
This product (SKU: RPCB0006) is produced using advanced expression systems and features a C-His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 21.39 kDa with an observed molecular weight of 20-25 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE.. Functional bioactivity has been validated through rigorous quality control assays, confirming its suitability for demanding research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Human Lipocalin-2/NGAL/LCN2 Protein |
| SKU: | RPCB0006 |
| Size: | 10 μg , 20 μg , 50 μg , 100 μg |
| Reactivity: | Human |
| Synonyms: | LCN2, HNL, NGAL, Neutrophil gelatinase-associated lipocalin, NGAL, 25 kDa alpha-2-microglobulin-related subunit of MMP-9, Lipocalin-2, Oncogene 24p3, Siderocalin, p25 |
| Tag: | C-His |
| Calculated MW: | 21.39 kDa |
| Observed MW: | 20-25 kDa |
| Gene ID: | 3934 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Human Lipocalin-2/NGAL/LCN2 Protein (RPCB0006), tested reactivity in HEK293 cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 0.01 EU/μg of the protein by LAL method |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Lyophilized from a 0.22 μm filtered solution of PBS, pH 7.4. |
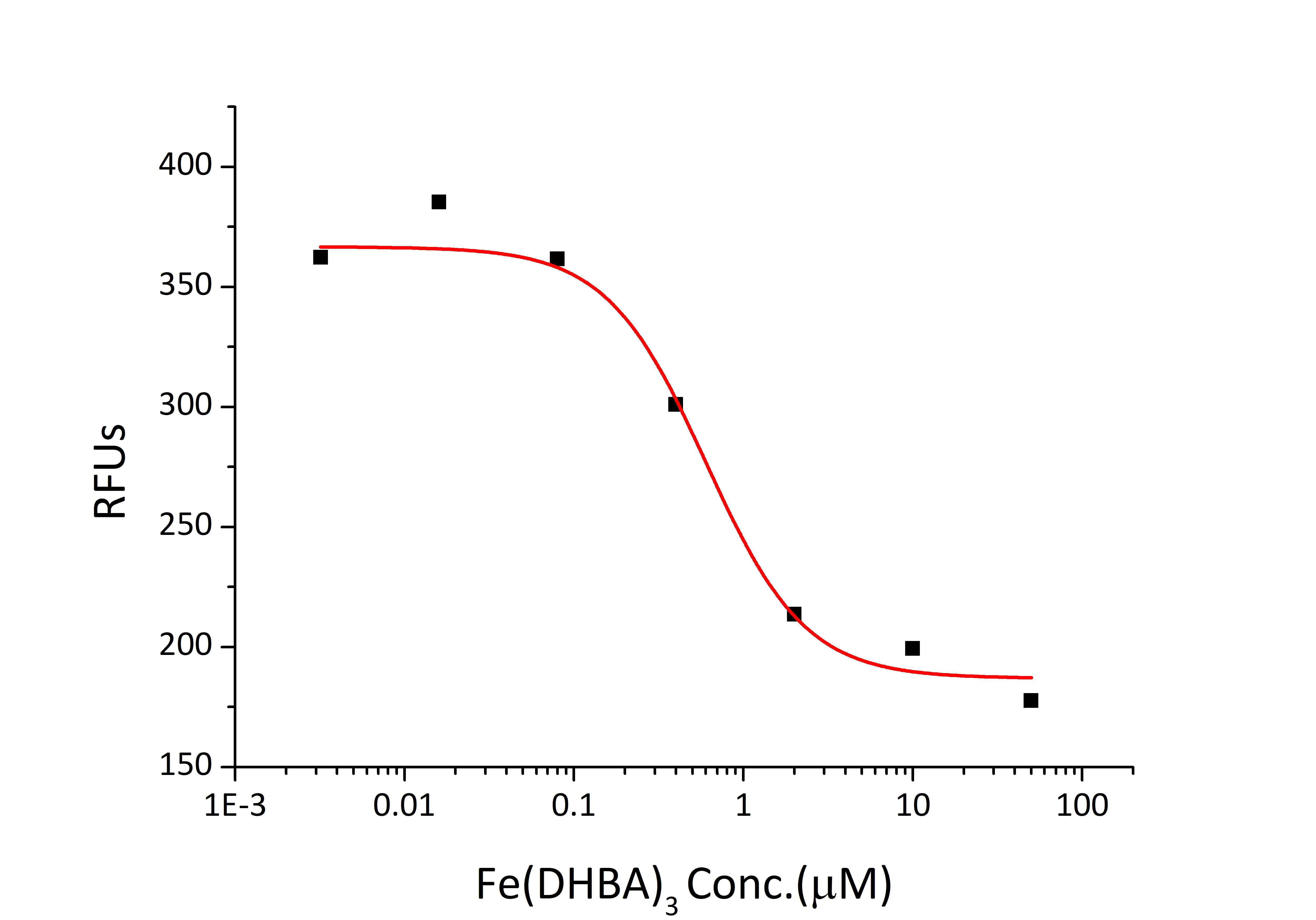
| Bio-Activity: | Measured by its ability to bind Iron(III) dihydroxybenzoic acid [Fe(DHBA)3]. The binding of Fe(DHBA)3 results in the quenching of Trp fluorescence in recombinant mouse Lipocalin-2. Recombinant human Lipocalin-2 can bind >0.60 μM of Fe(DHBA)3. |
| Reconstitution: | Centrifμge the vial before opening. Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Avoid vortex or vigorously pipetting the protein. For long term storage, it is recommended to add a carrier protein or stablizer (e.g. 0.1% BSA, 5% HSA, 10% FBS or 5% Trehalose), and aliquot the reconstituted protein solution to minimize free-thaw cycles. |
| Storage: | Store at -20℃. Store the lyophilized protein at -20℃ to -80℃ up to 1 year from the date of receipt. After reconstitution, the protein solution is stable at -20℃ for 3 months, at 2-8℃ for up to 1 week. |
Lipocalin-2, also known as Neutrophil Gelatinase-Associated Lipocalin (NGAL), was originally identified as a component of neutrophil granules. It is a 25 kDa protein existing in monomeric and homo- and heterodimeric forms, the latter as a dimer with human neutrophil gelatinases (MMP-9). Its expression has been observed in most tissues normally exposed to microorganism, and its synthesis is induced in epithelial cells during inflammation. Lipocalin-2 has been implicated in a variety of processes including cell differentiation, tumorigenesis, and apoptosis. Studies indicate that Lipocalin-2 binds a bacterial catecholate sidropore bound to ferric ion such as enterobactin with a subnanomolar dissociation constant. The bound ferric enterobactin complex breaks down slowly in a month into dihydroxybenzoyl serine and dihydroxybenzoic acid (DHBA). It also binds to a ferric DHBA complex with much less Kd values (7.9 nM). Secretion of Lipocalin‑2 in immune cells increases by stimulation of Toll-like receptor as an acute phase response to infection. As a result, it acts as a potent bacteriostatic reagent by sequestering iron. Moreover, Lipocalin-2 can alter the invasive and metastatic behavior of Ras-transformed breast cancer cells in vitro and in vivo by reversing epithelial to mesenchymal transition inducing activity of Ras, through restoration of E-cadherin expression, via effects on the Ras-MAPK signaling pathway.