Description
Recombinant Influenza A H1N1 (A/Puerto Rico/8/1934) Hemagglutinin / HA Protein
The Recombinant Influenza A H1N1 (A/Puerto Rico/8/1934) Hemagglutinin / HA Protein is a high-quality recombinant protein produced for advanced research applications in molecular biology and biotechnology. This protein serves as a critical reagent in various experimental contexts, including functional studies, binding assays, and therapeutic development programs, providing researchers with a standardized and reliable tool for investigating protein function and interactions.
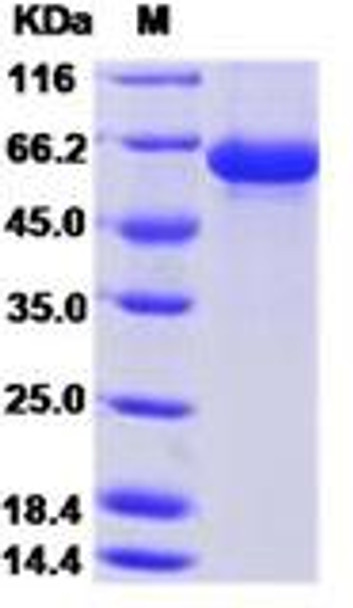
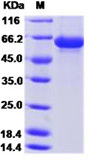
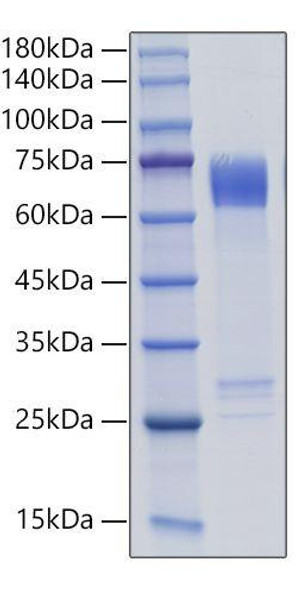
This product (SKU: RPCB0101) is produced using Baculovirus-Insect Cells and features a C-His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 59 kDa with an observed molecular weight of 60-66 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE.. Functional bioactivity has been validated through rigorous quality control assays, confirming its suitability for demanding research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Influenza A H1N1 (A/Puerto Rico/8/1934) Hemagglutinin / HA Protein |
| SKU: | RPCB0101 |
| Size: | 100 μg |
| Reactivity: | Influenza A |
| Synonyms: | Hemagglutinin, HA |
| Tag: | C-His |
| Expression Host: | Baculovirus-Insect Cells |
| Calculated MW: | 59 kDa |
| Observed MW: | 60-66 kDa |
| Gene ID: | 956529 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Influenza A H1N1 (A/Puerto Rico/8/1934) Hemagglutinin / HA Protein (RPCB0101), tested reactivity in Baculovirus-Insect Cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 1 EU/μg of the protein by LAL method. |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Lyophilized from sterile 20 mM Tris, 500 mM NaCl, 10 % glycerol, pH 8.0. Contact us for customized product form or formulation. |
| Bio-Activity: | Measured by its ability to agglutinate guinea pig red blood cells. HA titer is 0.02-0.4 μg/mL for 1% GRBC. |
| Reconstitution: | Centrifuge the vial before opening. Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Avoid vortex or vigorously pipetting the protein. For long term storage, it is recommended to add a carrier protein or stablizer (e.g. 0.1% BSA, 5% HSA, 10% FBS or 5% Trehalose), and aliquot the reconstituted protein solution to minimize free-thaw cycles. |
| Storage: | Store at -20℃.Store the lyophilized protein at -20℃ to -80 ℃ up to 1 year from the date of receipt. After reconstitution, the protein solution is stable at -20℃ for 3 months, at 2-8℃ for up to 1 week. |
The influenza viral Hemagglutinin (HA) protein is a homotrimer with a receptor binding pocket on the globular head of each monomer.HA has at least 18 different antigens. These subtypes are named H1 through H18.HA has two functions. Firstly, it allows the recognition of target vertebrate cells, accomplished through the binding to these cells' sialic acid-containing receptors. Secondly, once bound it facilitates the entry of the viral genome into the target cells by causing the fusion of the host endosomal membrane with the viral membrane. The influenza virus Hemagglutinin (HA) protein is translated in cells as a single protein, HA, or hemagglutinin precursor protein. For viral activation, hemagglutinin precursor protein (HA) must be cleaved by a trypsin-like serine endoprotease at a specific site, normally coded for by a single basic amino acid (usually arginine) between the HA1 and HA2 domains of the protein. After cleavage, the two disulfide-bonded protein domains produce the mature form of the protein subunits as a prerequisite for the conformational change necessary for fusion and hence viral infectivity.