The ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of samples in serum, plasma (EDTA, citrate, heparin).
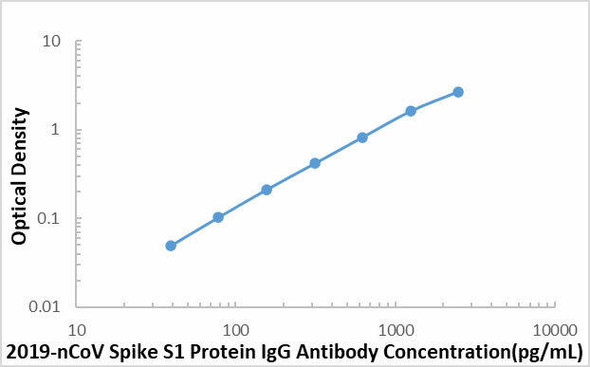
This SARS-COV-2 Spike S1 Protein ELISA kit is intended for laboratory research use only and not for use in diagnostic or therapeutic procedures. The stop solution changes color from blue to yellow and the intensity of the color is measured at 450 nm using a spectrophotometer. In order to measure the concentration of SARS-COV-2 Spike S1 Protein in the sample, this SARS-COV-2 Spike S1 Protein ELISA Kit includes a set of calibration standards. The calibration standards are assayed at the same time as the samples and allow the operator to produce a standard curve of optical density versus SARS-COV-2 Spike S1 Protein concentration. The concentration of the samples is then determined by comparing the O.D. of the samples to the standard curve.