Extracellular Vesicles in Neurodegenerative Disease: From Pathology to Therapeutic Potential
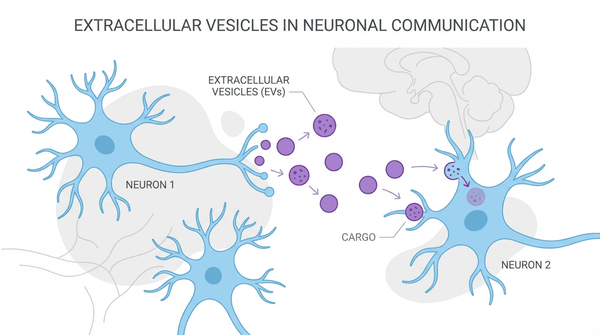
In the intricate landscape of the brain, cells constantly communicate, sending and receiving messages that govern our thoughts, memories, and movements. For decades, scientists believed this communication occurred primarily through direct synaptic connections. However, a new paradigm is emerging, centered on tiny messengers called extracellular vesicles (EVs). These microscopic packages, once dismissed as cellular debris, are now understood to be critical players in both health and disease. Groundbreaking research reveals that EVs facilitate the spreading of Lewy pathology between the peripheral and central nervous systems, fundamentally changing our understanding of how neurodegenerative diseases progress.
Introduction
Extracellular vesicles are lipid-bound particles released by virtually all cell types, carrying a diverse cargo of proteins, lipids, and nucleic acids. This cargo reflects the state of the parent cell, making EVs a rich source of biomarkers for diagnosing and monitoring disease. In the context of neurodegeneration, their ability to cross the blood-brain barrier has generated immense excitement. A 2024 review in Translational Neurodegeneration highlights that brain-derived EVs are being extensively investigated as biomarkers and therapeutic targets, offering a non-invasive window into the brain's health. This has profound implications for diseases like Alzheimer's and Parkinson's, where early and accurate diagnosis remains a major challenge.
The Dual Role of Extracellular Vesicles
Interestingly, EVs appear to play a dual role in neurodegenerative diseases, acting as both villains and heroes. On one hand, they contribute to the spread of toxic, misfolded proteins that are the hallmark of these conditions. On the other, they hold immense promise as therapeutic agents, capable of delivering protective molecules to damaged neurons. A recent study underscores this complexity, explaining that EVs can have a propagative detrimental effect or be exploited to deliver protective factors, posing a critical question: can vesicles be used to fight vesicle-propagated diseases?
Key Findings: Pathology and Promise
- Pathological Propagation: Research has shown that EVs can transport and transmit pathological proteins like α-synuclein and tau from cell to cell. A 2024 study found a substantial increase in filamentous α-synuclein within EVs in Parkinson's disease patients, suggesting they act as vehicles for disease progression. This prion-like propagation mechanism helps explain how the pathology spreads throughout the brain, leading to widespread neuronal damage.
- Biomarker Potential: The cargo of EVs offers a real-time snapshot of cellular health. A pivotal 2023 study in Brain demonstrated that neuronal extracellular vesicles serve as powerful biomarkers for cognitive impairment in Parkinson's disease. By analyzing the protein content of these vesicles, researchers could distinguish between patients with normal cognition and those with mild cognitive impairment or dementia, a critical step toward personalized medicine.
- Therapeutic Delivery: Perhaps the most exciting application of EVs is in therapy. Mesenchymal stem cell (MSC)-derived EVs, in particular, have shown remarkable therapeutic potential. A 2024 review highlights that MSC-derived EVs can cross the blood-brain barrier to deliver therapeutic cargo, modulating inflammation and promoting neuroregeneration. This opens the door to cell-free therapies that could repair damaged brain tissue.
Mechanisms of Action: How EVs Shape Neurodegeneration
To understand how EVs exert their effects, we must look at their cargo and how it interacts with recipient cells. When EVs released from a diseased neuron are taken up by a healthy one, they can transfer misfolded proteins, triggering a cascade of pathology. This process is central to the progression of many neurodegenerative disorders. Conversely, EVs derived from stem cells carry a cargo of growth factors, anti-inflammatory molecules, and microRNAs that can protect neurons from damage. These therapeutic EVs can reduce oxidative stress, inhibit apoptosis (programmed cell death), and promote the growth of new neurons and synapses.
Relevance to Human Health
The implications of EV research for human health are immense. The ability to diagnose neurodegenerative diseases early and non-invasively using a simple blood test would be a game-changer. A 2025 meta-analysis in npj Dementia confirmed that biomarkers from general EVs demonstrated superior diagnostic accuracy for Alzheimer's and related dementias. Furthermore, EV-based therapies offer a new frontier in treatment. By harnessing the natural healing properties of stem cell-derived EVs, we may be able to develop treatments that not only slow disease progression but also restore lost function.
Future Directions
Despite the rapid progress, several challenges remain. Standardizing methods for isolating and analyzing EVs is crucial for ensuring reliable and reproducible results. The aforementioned meta-analysis emphasized the importance of standardized EV isolation protocols to improve diagnostic accuracy. Additionally, more research is needed to understand the precise mechanisms by which EVs mediate their effects and to optimize their therapeutic potential. Future studies will focus on engineering EVs with specific cargo to target particular disease pathways and on developing scalable methods for producing clinical-grade EVs.
Conclusion
Extracellular vesicles have transformed our understanding of intercellular communication in the brain. From their role in propagating pathology to their promise as biomarkers and therapeutic agents, EVs are at the forefront of neurodegenerative disease research. As we continue to unravel the complexities of these tiny messengers, we move closer to a future where devastating diseases like Alzheimer's and Parkinson's can be diagnosed earlier, treated more effectively, and perhaps even prevented. The journey is just beginning, but the potential of extracellular vesicles to revolutionize neurology is undeniable.
References
- Wang L, et al. (2024). Extracellular vesicles: biological mechanisms and emerging therapeutic opportunities in neurodegenerative diseases. Translational Neurodegeneration. PMID: 39643909
- Scuteri A, Donzelli E. (2024). Dual role of extracellular vesicles in neurodegenerative diseases. World Journal of Stem Cells. PMID: 39734484
- Blommer J, et al. (2023). Extracellular vesicle biomarkers for cognitive impairment in Parkinson’s disease. Brain. PMID: 35833836
- Taha HB. (2025). Alzheimer’s disease and related dementias diagnosis: a biomarkers meta-analysis of general and CNS extracellular vesicles. npj Dementia. DOI: 10.1038/s44400-024-00002-y
- Xiong Y, Mahmood A, Chopp M. (2024). Mesenchymal stem cell-derived extracellular vesicles as a cell-free therapy for traumatic brain injury via neuroprotection and neurorestoration. Neural Regeneration Research. PMID: 37488843
- Ishiguro Y, et al. (2024). Extracellular vesicles contain filamentous alpha-synuclein and facilitate the propagation of Parkinson's pathology. Biochemical and Biophysical Research Communications. PMID: 38359614
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025