Description
| Product Name: | Chicken PICP (Procollagen I C-Terminal Propeptide) ELISA Kit |
| SKU: | AEKE03006 |
| Reactivity: | Chicken |
| Assay Type: | Sandwich ELISA |
| Sensitivity: | 1.71 ng/mL |
| Range: | 3.13-200 ng/mL |
| Sample Type: | Serum, Plasma, Tissue Homogenates, Cell Lysates, Cell Culture Supernates and Other Biological fluids |
| Component | Quantity | Storage | |
| 48T | 96T | ||
| Pre-Coated Microplate | 6 strips × 8 wells | 12 strips × 8 wells | 4 °C / −20 °C |
| Standard (Lyophilized) | 1 vial | 2 vials | 4 °C / −20 °C |
| Biotinylated Antibody (100×) | 60 μL | 120 μL | 4 °C / −20 °C |
| Streptavidin–HRP (100×) | 60 μL | 120 μL | 4 °C / −20 °C |
| Standard / Sample Diluent Buffer | 10 mL | 20 mL | 4 °C / −20 °C |
| Biotinylated Antibody Diluent | 6 mL | 12 mL | 4 °C / −20 °C |
| HRP Diluent | 6 mL | 12 mL | 4 °C / −20 °C |
| Wash Buffer (25×) | 10 mL | 20 mL | 4 °C / −20 °C |
| TMB Substrate Solution | 6 mL | 10 mL | 4 °C / −20 °C (store in the dark) |
| Stop Reagent | 3 mL | 6 mL | 4 °C / −20 °C |
| Plate Covers | 1 piece | 2 pieces | RT |
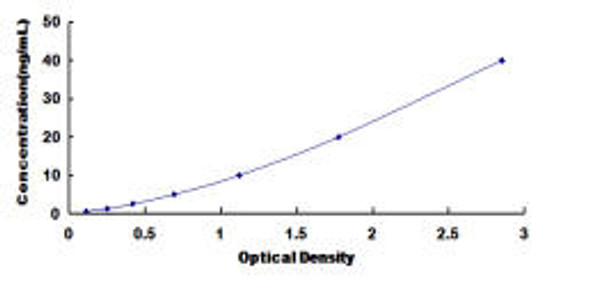
The assay is based on the sandwich enzyme immunoassay principle. The microtiter plate provided in this kit is pre-coated with an antibody specific for Chicken PICP. Standards or samples are added to the designated wells, followed by the addition of a biotin-conjugated antibody specific for Chicken PICP. Avidin conjugated to horseradish peroxidase (HRP) is then introduced and incubated. After addition of the TMB substrate solution, only wells containing Chicken PICP, the biotin-conjugated antibody, and the enzyme–avidin complex will develop a color reaction. The enzymatic reaction is terminated by the addition of sulfuric acid, and absorbance is measured spectrophotometrically at 450 nm ± 10 nm. The concentration of Chicken PICP in the samples is determined by comparing the optical density (OD) values of the samples against the standard curve.
| Standard Curve: |
|
|||||||||||||||||||||||||||
| Recovery: |
Matrices were spiked with defined levels of recombinant Chicken PICP, and recovery was determined by comparing measured concentrations with the expected values.
|
|||||||||||||||||||||||||||
| Linearity: |
Linearity was assessed by testing serial dilutions of spiked samples. Results are expressed as the percentage of the calculated concentration relative to the expected value.
|
|||||||||||||||||||||||||||
| Precision: |
Intra-assay Precision (within-run): Coefficient of variation (CV) < 8%
Inter-assay Precision (between-run): Coefficient of variation (CV) < 10% |
*Note: The following protocol is a general guideline. Please refer to the specific protocol provided with your kit, as procedures may vary slightly by lot.
| Step | Procedure |
| 1. | Equilibrate all kit components to room temperature. Add 100 μL of the Standard Working Solution (prepared by serial dilution as described) or 100 μL of sample to each well. Incubate at 37 °C for 80 minutes. |
| 2. | Discard the liquid from each well. Wash the wells three times with 200 μL of 1× Wash Buffer per well. After the final wash, blot the plate gently onto clean absorbent paper. Add 100 μL of the 1× Biotinylated Antibody Working Solution to each well and incubate at 37 °C for 50 minutes. |
| 3. | Discard the liquid from the wells and repeat the washing step three times with 200 μL of 1× Wash Buffer per well. Blot dry. Add 100 μL of the 1× Streptavidin–HRP Working Solution to each well and incubate at 37 °C for 50 minutes. |
| 4. | Discard the liquid from the wells. Wash the plate five times with 200 μL of 1× Wash Buffer per well. Blot dry. Add 90 μL of TMB Substrate Solution to each well and incubate at 37 °C for 20 minutes in the dark. |
| 5. | Add 50 μL of Stop Solution to each well. Mix thoroughly on a plate shaker for 1 minute. Measure absorbance immediately at 450 nm. |
Proper sample preparation is critical for achieving reliable and reproducible ELISA results. The following are recommended protocols for various sample types.
| Sample Type | Preparation Protocol |
| Serum | Collect blood into a serum separator tube. Allow samples to clot for 2 hours at room temperature or overnight at 4 °C. Centrifuge at 1000 × g for 20 minutes. Assay freshly prepared serum immediately, or store aliquots at −20 °C or −80 °C. Avoid repeated freeze–thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge at 1000 × g for 15 minutes at 2–8 °C within 30 minutes of collection. Remove plasma and assay immediately, or store aliquots at −20 °C or −80 °C. Avoid repeated freeze–thaw cycles. |
| Tissue Homogenates |
Preparation may vary depending on tissue type: 1. Rinse tissues with pre-cooled PBS to remove blood and weigh. 2. Mince into small pieces and homogenize in fresh lysis buffer (PBS is suitable for most tissues; select buffer based on target protein localization) at a ratio of 1:9 (w:v) (e.g., 900 μL buffer per 100 mg tissue) using a glass homogenizer on ice. 3. Sonicate (ultrasonicate) until the suspension becomes clear. 4. Centrifuge at 10,000 × g for 5 minutes. Collect the supernatant for immediate assay, or store aliquots at ≤ −20 °C. Note: It is recommended to measure protein concentration (e.g., using BCA Protein Assay, Product BC016) to normalize results per mg of protein. |
| Cell Lysates |
1. For adherent cells, wash gently with pre-cooled PBS, detach with trypsin, and collect by centrifugation at 1000 × g for 5 minutes.
Suspension cells may be collected directly by centrifugation. 2. Wash cells three times with pre-cooled PBS. 3. Resuspend in fresh lysis buffer at a concentration of 1 × 107 cells/mL. If necessary, sonicate until the lysate is clear. 4. Centrifuge at 1500 × g for 10 minutes at 2–8 °C. Collect the supernatant for immediate use or store aliquots at ≤ −20 °C. |
| Urine | Collect first-morning midstream urine directly into a sterile container. Centrifuge to remove debris. Assay immediately or store aliquots at ≤ −20 °C. Avoid repeated freeze–thaw cycles. |
| Saliva | Collect using a saliva collection device or equivalent. Centrifuge at 1000 × g for 15 minutes at 2–8 °C to remove particulates. Assay immediately or store aliquots at ≤ −20 °C. Avoid repeated freeze–thaw cycles. |
| Feces | Collect at least 50 mg of dried feces. Wash three times with PBS at a ratio of 1:9 (w:v) (e.g., 900 μL PBS per 100 mg feces). Sonicate (or grind) thoroughly, then centrifuge at 5000 × g for 10 minutes. Collect the supernatant for testing. |
| Cell Culture Supernatants and Other Biological Fluids | Centrifuge at 1000 × g for 20 minutes. Collect the supernatant and assay immediately, or store aliquots at −20 °C or −80 °C. Avoid repeated freeze–thaw cycles. |
| Cerebrospinal Fluid (CSF) | Remove particulates by centrifugation. Assay immediately, or store aliquots at ≤ −20 °C. Avoid repeated freeze–thaw cycles. |