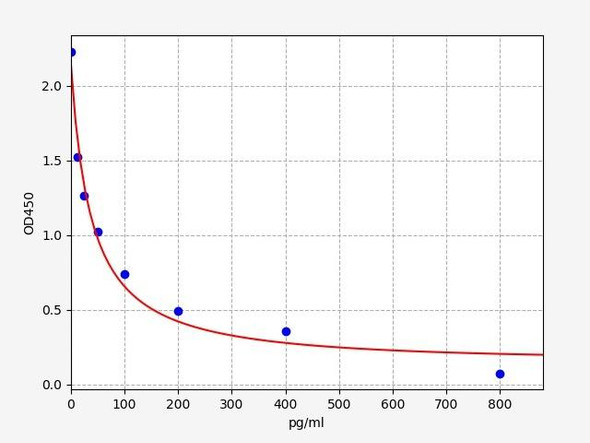
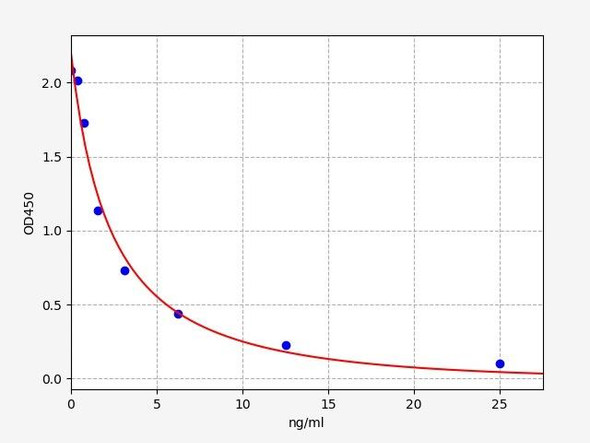
Enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of free Ranibizumab (Lucentis®) in aqueous humour. The Assay Genie Ranibizumab ELISA has been specially developed for the quantitative analysis of free Ranibizumab in aqueous humour and is for research use only.
Description
Ranibizumab (Lucentis®) ELISA Kit
Ranibizumab (Lucentis®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples are incubated in the microtitre plate coated with the reactant for ranibizumab (Lucentis®). After incubation, the wells are washed. A horse radish peroxidase (HRP) conjugated probe is added and binds to ranibizumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of ranibizumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Ranibizumab (Lucentis®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 70 |
Sample Type | Aqueous humour |
Number of Assays | 96 |
Detection Limit (ng/mL) | 0.3 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-VEGF-A mAb Lucentis |
Ranibizumab ELISA Kit
Ranibizumab (Lucentis®) mode of action
Ranibizumab is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment designed for intraocular use. Ranibizumab binds to and inhibits the biologic activity of human vascular endothelial growth factor A (VEGF-A). Ranibizumab has a molecular weight of approximately 48 kilodaltons and is produced by an E. coli expression system in a nutrient medium containing the antibiotic tetracycline (tetracycline is not detectable in the final product).
Ranibizumab (Lucentis®) uses
Ranibizumab is marketed under the name Lucentis®. It is indicated for the treatment of macular edema after retinal vein occlusion, age-related macular degeneration (wet), and diabetic macular edema. Ranibizumab binds to the receptor binding site of active forms of VEGF-A, including the biologically active, cleaved form of this molecule, VEGF110. The binding of ranibizumab to VEGF-A prevents the interaction of VEGFA with its receptors (VEGFR1 and VEGFR2) on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation.
Ranibizumab is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment designed for intraocular use. Ranibizumab is a VEGF-A antagonist that binds to and inhibits the biologic activity of active forms of human VEGF-A, including the cleaved form (VEGF110). VEGF-A has been shown to cause neovascularization (angiogenesis) and an increase in vascular permeability, which is thought to contribute to the progression of the neovascular form of age-related macular degeneration (AMD).
Ranibizumab (Lucentis®) treatment
Following monthly intravitreal injections, maximum aqueous humour concentrations are minimal around 0.3 ng/mL to 2.36 ng/mL. The most common toxic effects to the eye are eye pain, vitreous floaters, increased intraocular pressure, conjunctival hemorrhage, intraocular inflammation, and foreign body sensation. Also, arterial thromboembolic events have occurred in patients.
Ranibizumab (Lucentis®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
7 x 0.3 mL | Ranibizumab Standards A-F (10X), High Level Control (10X), Low Level Control (10X) 300; 100; 30; 10; 3; 0 nanogram/mL |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Ranibizumab (Lucentis®) ELISA Protocol
| Steps | Protocol |
1 | Dilute each of the standards and samples (aqueous humour) using Assay Buffer as described in “Dilution of Standards and Samples (aqueous humour)” section of the technical manual |
2 | Pipette 100µl of each Diluted Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
3 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
LUCENTIS is a trademark of GENENTECH, INC