Description
Recombinant Human Cathepsin B Protein
The Recombinant Human Cathepsin B Protein is a biologically active recombinant protein that plays a significant role in various cellular processes and signaling pathways in human biology. This protein is widely employed in immunological research, cell biology studies, protein-protein interaction analyses, and therapeutic development, providing researchers with a reliable tool for investigating Cathepsin B function and its implications in health and disease.
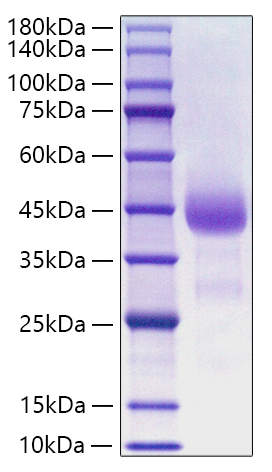
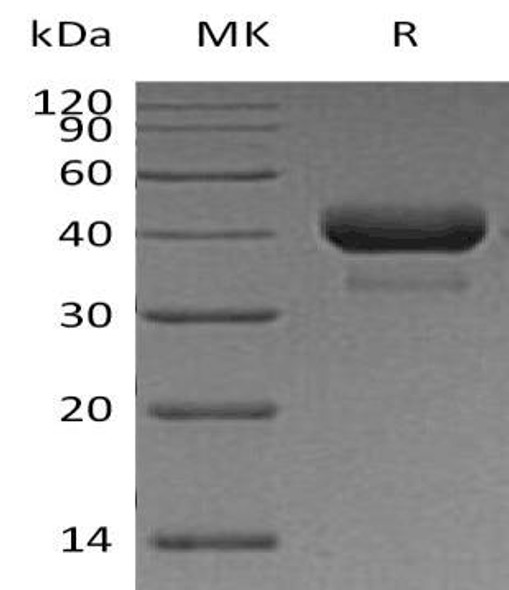
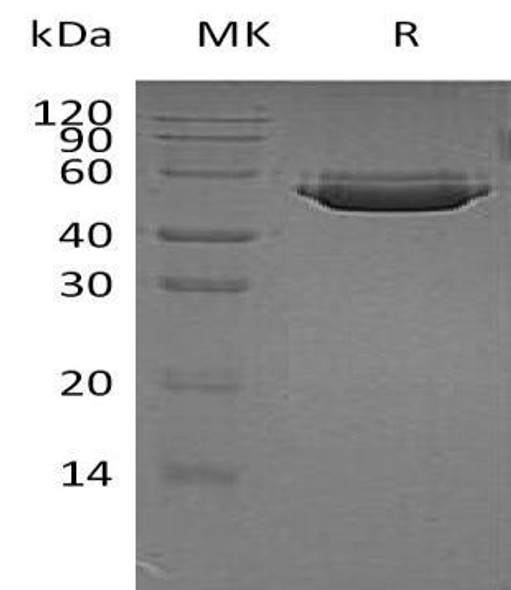
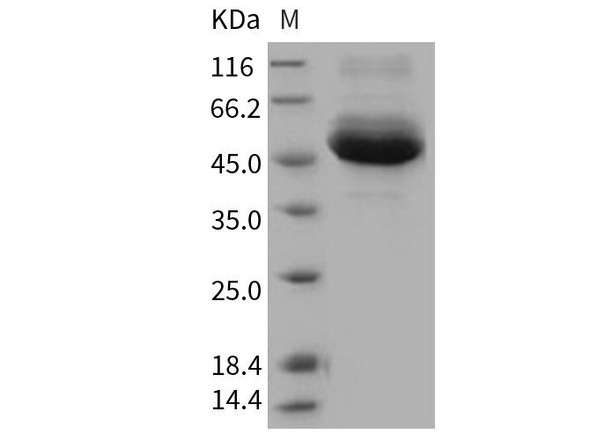
This product (SKU: RPCB1791) is produced using HEK293 Cells and features a C-His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 36.74 kDa with an observed molecular weight of 40-50 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE.. Functional bioactivity has been validated through rigorous quality control assays, confirming its suitability for demanding research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Human Cathepsin B Protein |
| SKU: | RPCB1791 |
| Size: | 10 μg , 20 μg , 50 μg , 100 μg |
| Reactivity: | Human |
| Synonyms: | Cathepsin B, 3.4.22.1, APP secretase, APPS, Cathepsin B1, CTSB |
| Tag: | C-His |
| Expression Host: | HEK293 Cells |
| Calculated MW: | 36.74 kDa |
| Observed MW: | 40-50 kDa |
| Gene ID: | 1508 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Human Cathepsin B Protein (RPCB1791), tested reactivity in HEK293 Cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 0.1 EU/μg of the protein by LAL method. |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Lyophilized from a 0.22 μm filtered solution of PBS, pH 7.4. |
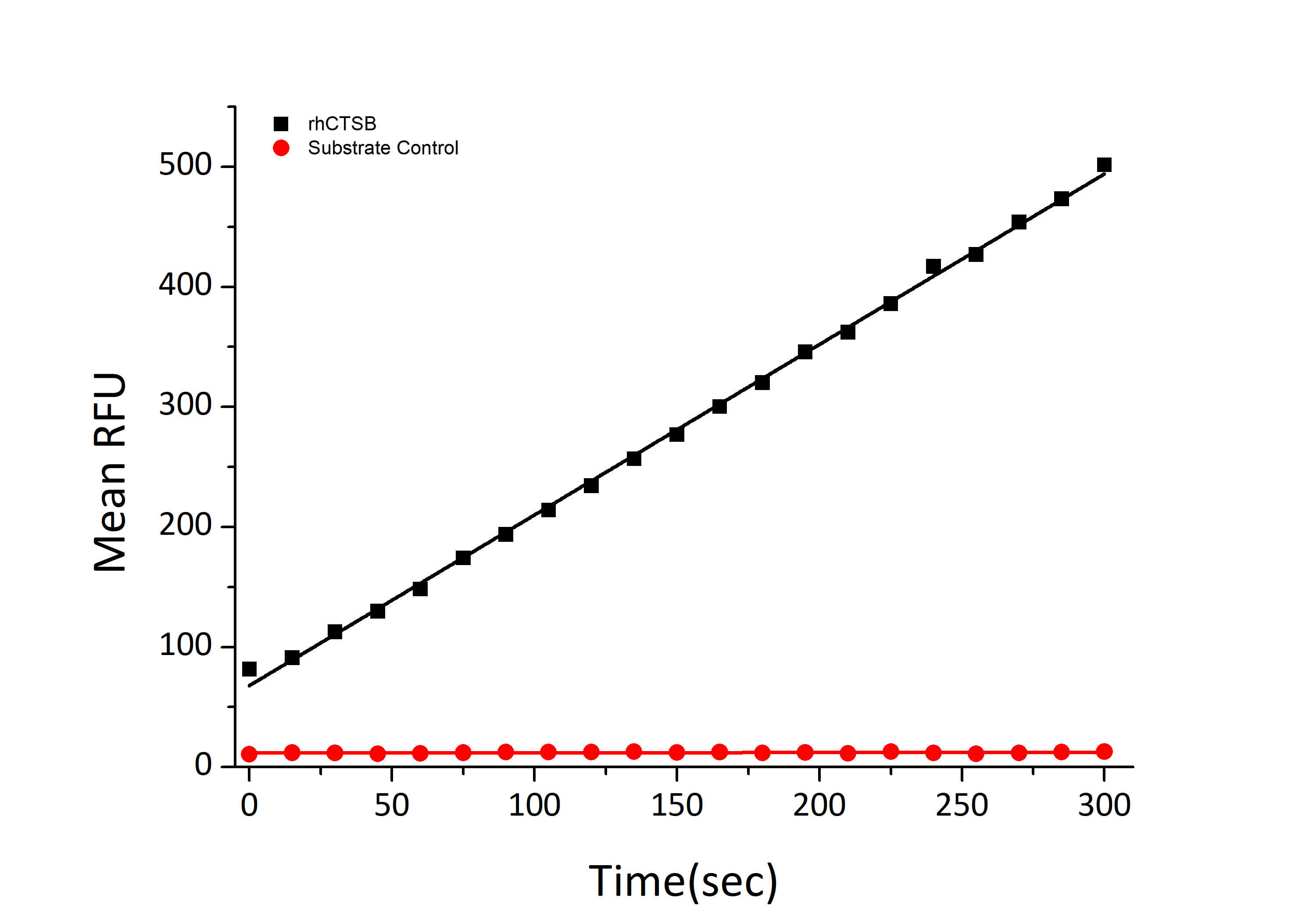
| Bio-Activity: | Measured by its ability to cleave the fluorogenic peptide substrate Z-LR-AMC (R&D Systems, Catalog # ES008). The specific activity is >3468 pmoles/min/μg. |
| Reconstitution: | Centrifuge the vial before opening. Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Avoid vortex or vigorously pipetting the protein. For long term storage, it is recommended to add a carrier protein or stablizer (e.g. 0.1% BSA, 5% HSA, 10% FBS or 5% Trehalose), and aliquot the reconstituted protein solution to minimize free-thaw cycles. |
| Storage: | Store at -20℃.Store the lyophilized protein at -20℃ to -80 ℃ up to 1 year from the date of receipt. After reconstitution, the protein solution is stable at -20℃ for 3 months, at 2-8℃ for up to 1 week. |
Cathepsin B is a papain-family cysteine protease that is normally located in lysosomes, where it is involved in the turnover of proteins and plays various roles in maintaining the normal metabolism of cells. This protease has been implicated in pathological conditions, e.g., tumor progression and arthritis. In disease conditions, increases in the expression of cathepsin B occur at both the gene and protein levels. Cathepsin B is synthesized as a preproenzyme and the primary pathways for its normal trafficking to the lysosome utilize mannose 6-phosphate receptors (MPRs). Mature cathepsin B has the ability to degrade several extracellular matrix components at both neutral and acidic pH and has been implicated in the progression of several human and rodent tumors progression and arthritis. Cathepsin B expression is increased in many human cancers at the mRNA, protein and activity levels. It is also frequently overexpressed in premalignant lesions, an observation that associates this protease with local invasive stages of cancer. Increased expression of cathepsin B in primary cancers, and especially in preneoplastic lesions, suggests that this enzyme might have pro-apoptotic features. Active cathepsin B is also secreted from tumours, a mechanism likely to be facilitated by lysosomal exocytosis or extracellular processing by surface activators. Cathepsin B is localized to caveolae on the tumour surface, where binding to the annexin II heterotetramer occurs. Thus CTSB is suggested as a tumor marker. Additionally, Cathepsin B can degrade extracellular matrix proteins, such as collagen IV and laminin, and can activate the precursor form of urokinase plasminogen activator (uPA), perhaps thereby initiating an extracellular proteolytic cascade.