Description
Recombinant Human MetAP1 Protein
The Recombinant Human MetAP1 Protein is a biologically active recombinant protein that plays a significant role in various cellular processes and signaling pathways in human biology. This protein is widely employed in immunological research, cell biology studies, protein-protein interaction analyses, and therapeutic development, providing researchers with a reliable tool for investigating MetAP1 function and its implications in health and disease.
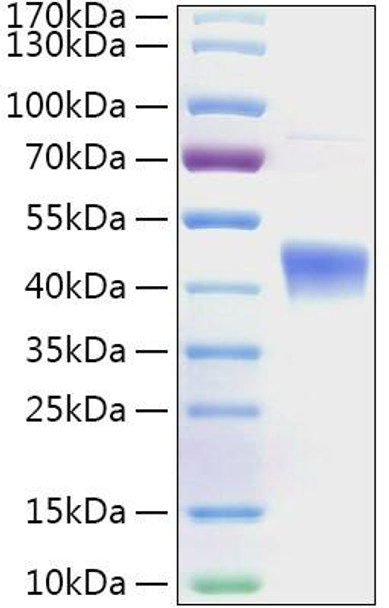
This product (SKU: RPCB2001) is produced using E.coli and features a No tag tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 43.2 kDa with an observed molecular weight of 40-50 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE., ensuring exceptional quality and consistency for research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Human MetAP1 Protein |
| SKU: | RPCB2001 |
| Size: | 50 μg |
| Reactivity: | Human |
| Synonyms: | Methionine aminopeptidase 1, MAP 1, MetAP 1, Peptidase M 1, METAP1 |
| Tag: | No tag |
| Expression Host: | E.coli |
| Calculated MW: | 43.2 kDa |
| Observed MW: | 40-50 kDa |
| Gene ID: | 23173 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Human MetAP1 Protein (RP03202LQ), tested reactivity in E.coli and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 1 EU/μg of the protein by LAL method. |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Supplied as a 0.2 μm filtered solution of 20 mM Tris-HCl, 500 mM NaCl, 10% Glycerol, pH 8.0. |
| Storage: | Store at -70℃. This product is stable at ≤ -70℃ for up to 1 year from the date of receipt. For optimal storage, aliquot into smaller quantities after centrifugation and store at recommended temperature. Avoid repeated freeze-thaw cycles. |
Methionine Aminopeptidase 1 is a member of the M24 family of metalloproteases. METAP1 plays an important role in G(2)/M phase regulation of the cell cycle and may serve as a promising target for the discovery and development of new anticancer agents. METAP1 and METAP2 have different substrate specificity due to the differences in both size and shape of the active sites. The proteolytic removal of N-terminal methionine from nascent peptides is catalyzed by a family of enzymes known as methionine aminopeptidases (MetAPs) and is essential for cell growth. Inhibition of METAPs provides a novel strategy in developing anti-cancer drugs.