Description
Recombinant Human Serum Albumin (rHSA) Protein - Excipient-Grade
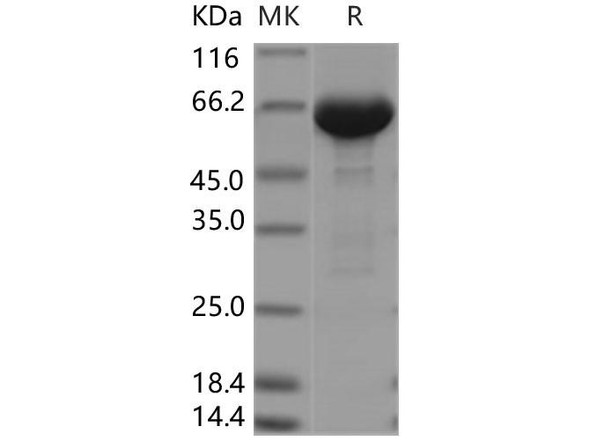
rHSA is a high-purity, excipient-grade recombinant human serum albumin (rHSA) produced in Oryza sativa (rice endosperm). Developed as a superior alternative to bovine serum albumin (BSA), rHSA is animal-free, virus-free, and engineered for unmatched quality and consistency in sensitive applications.
As the most abundant protein in human plasma, albumin plays a vital role in osmotic regulation, pH buffering, and molecular transport. rHSA delivers these same physiological functions with enhanced safety and reproducibility, without the risks associated with animal-derived products. Compared with fetal bovine serum (FBS), bovine serum albumin (BSA), and plasma-derived albumin (pHSA), plant-derived HSA has higher purity and better batch stability.
| ✓ | Ultra-Pure | >99.99% purity |
| ✓ | Animal-Free | No risk of prions or blood-borne pathogens |
| ✓ | Highly Consistent | Stable across batches for reliable performance |
| ✓ | Superior Safety Profile |
|
| ✓ | pH Range | 6.4–7.4 |
| Product Name: | Recombinant Human Serum Albumin (rHSA) Protein - Excipient-Grade |
| SKU: | RPES7198 |
| Specification: |
|
| Recommended Applications: |
|
| Excipient grade | Cell culture grade | |
| Specification | 50 ml / bottle Purity: 20% |
Lyophilized powder, 100 g / bottle |
| Purity | >99.99% | >99% |
| Endotoxin | <1.67 EU/mL | <0.125 EU/mg |
| pH | 6.4–7.4 | 6.0–8.0 |
| Host cell protein (HCP) | <5 µg/g | Not tested |
| Host cell DNA (HCD) | <0.5 ng/g | Not tested |
| Aluminum residue | 200 µg/L | Not tested |
| Ammonium sulfate residue | 0.5 g/L | Not tested |
| Recommended Applications | Vaccine protective agent, drug carrier, fusion drug cell protective agent, medical device embedding agent, cell culture | Cell culture, plasma matrix control, blocking agent, enzyme protective agent |