Description
Recombinant Mouse IL-27A Protein
The Recombinant Mouse IL-27A Protein is a high-quality recombinant protein designed for murine biological research applications. This protein serves as an essential reagent in mouse model studies, comparative immunology research, and preclinical therapeutic evaluations, enabling scientists to investigate IL-27A biology and its relevance to human disease mechanisms through translational research approaches.
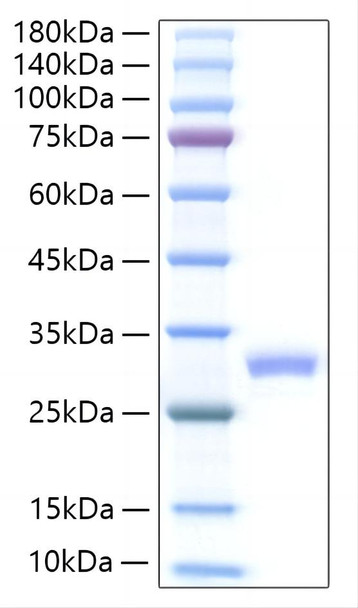
This product (SKU: RPCB1257) is produced using HEK293 cells and features a C-His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 24.26 kDa with an observed molecular weight of 25-30 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE., ensuring exceptional quality and consistency for research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Mouse IL-27A Protein |
| SKU: | RPCB1257 |
| Size: | 10 μg , 20 μg , 50 μg , 100 μg |
| Reactivity: | Mouse |
| Synonyms: | IL27 p28, IL-27 p28, IL27, IL-27, IL27A, IL-27A, IL27p28, IL-27p28, IL30, p28, IL-30 |
| Tag: | C-His |
| Expression Host: | HEK293 cells |
| Calculated MW: | 24.26 kDa |
| Observed MW: | 25-30 kDa |
| Gene ID: | 246779 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Mouse IL-27A Protein (RPCB1257), tested reactivity in HEK293 cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 0.01 EU/μg of the protein by LAL method |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Lyophilized from a 0.22 μm filtered solution of PBS, pH 7.4. |
| Reconstitution: | Centrifuge the vial before opening. Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Avoid vortex or vigorously pipetting the protein. For long term storage, it is recommended to add a carrier protein or stablizer (e.g. 0.1% BSA, 5% HSA, 10% FBS or 5% Trehalose), and aliquot the reconstituted protein solution to minimize free-thaw cycles. |
| Storage: | Store at -20℃.Store the lyophilized protein at -20℃ to -80 ℃ up to 1 year from the date of receipt. After reconstitution, the protein solution is stable at -20℃ for 3 months, at 2-8℃ for up to 1 week. |
Interleukin‑27 p28, also known as IL‑30, is a secreted 28 kDa protein that is considered a member of the IL‑6/IL‑12 interfamily cytokine family. p28/IL‑30 is one of several alpha ‑chain proteins that can associate with various beta ‑chain proteins to form heterodimeric cytokines in these families. The alpha -chains (e.g. IL‑6, IL‑11, Cardiotrophin‑1, CLC/CNTF, LIF, Oncostatin M, IL‑23 p19, IL‑27 p28/IL‑30, IL‑12/IL‑35 p35) have a four‑helix bundle structure, while the beta ‑chains (e.g. EBI‑3, CLF‑1, IL‑12/IL‑23 p40) resemble class 1 cytokine receptors. These cytokines utilize heteromeric cell surface receptors which contain shared as well as ligand‑specific subunits. Divergent biological responses are obtained from the combinatorial association of cytokine subunits and their interaction with various combinations of receptor subunits. Complexity in this system is increased by the generation of soluble receptors and by the competition between proteins for shared subunit pairing. p28/IL‑30 is expressed by macrophages and dendritic cells, and is up‑regulated in these cells by inflammatory stimuli. It was first described as a partner with EBI‑3 in the heterodimeric cytokine IL‑27. IL‑27 signals through a receptor complex composed of IL‑27 R alpha /WSX-1/TCCR and gp130. This interaction enhances the proinflammatory activation of naïve CD4+ T cells, NK cells, mast cells, and monocytes and the cytotoxic activity of CD8+ T cells. IL‑27 also exhibits anti-inflammatory activity, including the induction of IL‑10 production by naïve and memory T cells, the activation of regulatory T cells (Treg), and the suppression of Th17 cytokine secretion. Alternatively, p28/IL‑30 associates with CLF‑1 to create a cytokine that triggers responses through IL‑27 R alpha, IL‑6 R alpha, and gp130. Like IL‑27, p28‑CLF-1 heterodimers co‑stimulate IFN‑ gamma production by NK cells, and induce IL‑10 secretion by CD4+ T cells. In contrast to IL‑27, however, p28‑CLF-1 is reported to promote the differentiation of Th17 cells. A third mode of p28 action enables it to stimulate cells that express both IL‑6 R alpha and gp130, but lack IL‑27 R alpha. Similar to the IL‑6 system, the presence of IL‑6 R alpha on the cell surface is not even required if p28/IL‑30 associates with a soluble form of IL‑6 R alpha. This combination can trigger trans signaling through gp130, a mechanism that has been demonstrated for complexes of IL‑6 with soluble IL‑6 R alpha. Over‑expression of p28/IL‑30 in vivo interferes with humoral antibody responses and protects from IL‑12 induced liver inflammation.