Alternative Complement Pathway: Activation, Regulation, and Significance
As a vital component of the immune system, the Alternative Complement Pathway plays a crucial role in defending the body against pathogens and maintaining immune homeostasis. In this blog, we will explore the underlying mechanisms of its activation, examine the key components involved, and shed light on its intricate biological functions.
Key Takeaways:
- The Alternative Complement Pathway is crucial for immune defense, activating spontaneously or in response to pathogens.
- It contributes to inflammation, opsonization, and the clearance of immune complexes.
- Regulated by proteins like factor H and I to prevent damage to healthy cells.
- Dysregulation of this pathway is linked to diseases like atypical hemolytic uremic syndrome and age-related macular degeneration.
- Research into its mechanisms is key for developing targeted immunological therapies.
Table of Contents
Jump to a section:
Alternative Complement Pathway
The complement system (CS), first identified by Bordet in 1896, is the crucial component uniting the innate and adaptive immune systems. It is able to distinguish between healthy cells and foreign or damaged cells, which allows it to play a crucial part in defending against pathogens through opsonization of bacterial and viral cells, chemotaxis, the start of an inflammatory response at the site of infection, the elimination of modified or damaged cells, and the dissolution of immune complexes. The three pathways—classical (CP), alternative (AP), and lectin (LP)—can activate the complement in humans.
The Alternative Complement Pathway is a critical component of the immune system, functioning alongside the classical and lectin pathways to combat pathogens and maintain immune homeostasis. It is an intricate cascade of biochemical reactions that becomes activated in response to various triggers.
At its core, the Alternative Complement Pathway acts as a surveillance system, constantly patrolling the body for foreign or aberrant cells. Unlike the classical pathway, which is antigen-dependent, the alternative pathway can be initiated spontaneously, making it an essential first line of defense against infections.
Complement Pathways
Exploring the Alternative Pathway in Complement Activation
The activation of the Alternative Complement Pathway is a finely orchestrated process that plays a critical role in immune defense. The activation of the alternative pathway can occur through both spontaneous and pathogen-induced mechanisms.
In the absence of any external triggers, the alternative pathway can be spontaneously activated through the hydrolysis of C3 molecules. This process results in the generation of C3b, which serves as a central component in initiating the cascade of complement activation.
Pathogens, such as bacteria or viruses, can directly or indirectly trigger the activation of the alternative complement pathway. Various pathogen-associated molecular patterns (PAMPs) present on the surface of microorganisms can interact with complement components, leading to pathway activation. For example, certain bacterial cell wall components, such as lipopolysaccharides (LPS), can directly bind to C3b and initiate complement activation.
Once the alternative complement pathway is activated, a series of events takes place. The binding of C3b to pathogen surfaces or other activating surfaces leads to the assembly of C3 convertase enzymes. These enzymes, composed of C3b and factors B and D, cleave additional C3 molecules into C3a and C3b, amplifying the complement response. The deposition of C3b on the pathogen surface promotes opsonization, marking the target for recognition and elimination by phagocytic cells. Additionally, the release of C3a during the cleavage of C3 induces inflammation by recruiting immune cells and activating mast cells. The C3 convertase enzymes formed during the activation process further amplify the cascade by generating more C3b molecules. This positive feedback loop enhances the immune response and contributes to the elimination of pathogens. The alternative complement pathway is interconnected with the terminal pathway, where the cleavage of C5 leads to the assembly of the membrane attack complex (MAC). MAC formation results in the lysis of pathogens, contributing to their destruction.
Alternative Pathway
Role and Regulation of the Alternative Complement Pathway
One of the primary roles of the Alternative Complement Pathway is to enhance the inflammatory response. Upon activation, it generates a cascade of enzymatic reactions, resulting in the production of anaphylatoxins, such as C3a and C5a. These anaphylatoxins contribute to local inflammation, attract immune cells to the site of infection, and promote the clearance of pathogens. Additionally, the Alternative Complement Pathway aids in opsonization, a process by which pathogens are marked for phagocytosis. The activation of the pathway leads to the deposition of C3b on the surface of pathogens, facilitating their recognition and engulfment by phagocytic cells. Furthermore, the Alternative Complement Pathway participates in the clearance of immune complexes and apoptotic cells. It promotes the recognition and removal of these cellular debris, preventing the accumulation of potentially harmful materials and maintaining tissue homeostasis.
To prevent excessive complement activation and potential self-damage, the Alternative Complement Pathway is tightly regulated by various control mechanisms. One crucial regulator is the protein factor H, which acts as a cofactor for factor I. Factor H binds to host cell surfaces and helps prevent the inadvertent deposition of C3b. This regulatory function ensures that complement activation occurs primarily on foreign surfaces, limiting damage to healthy host cells. Additionally, factor I cleaves and inactivates C3b, preventing the amplification of the complement cascade. This cleavage process, in conjunction with factor H, helps maintain the delicate balance between complement activation and regulation. Another key regulatory mechanism involves membrane-bound complement regulators, such as membrane cofactor protein (MCP) and decay-accelerating factor (DAF). These regulators are expressed on host cell surfaces and serve to inhibit complement activation by destabilizing the C3 convertase enzyme complex. Furthermore, several soluble proteins, including clusterin and vitronectin, participate in the regulation of the Alternative Complement Pathway by binding to activated complement components and preventing their excessive accumulation.
The precise interplay of these regulatory mechanisms ensures that the Alternative Complement Pathway responds appropriately to infections while avoiding unnecessary damage to self-tissues.
Alternative Complement Pathway Related Products
Alternative Pathway Related ELISA Kits
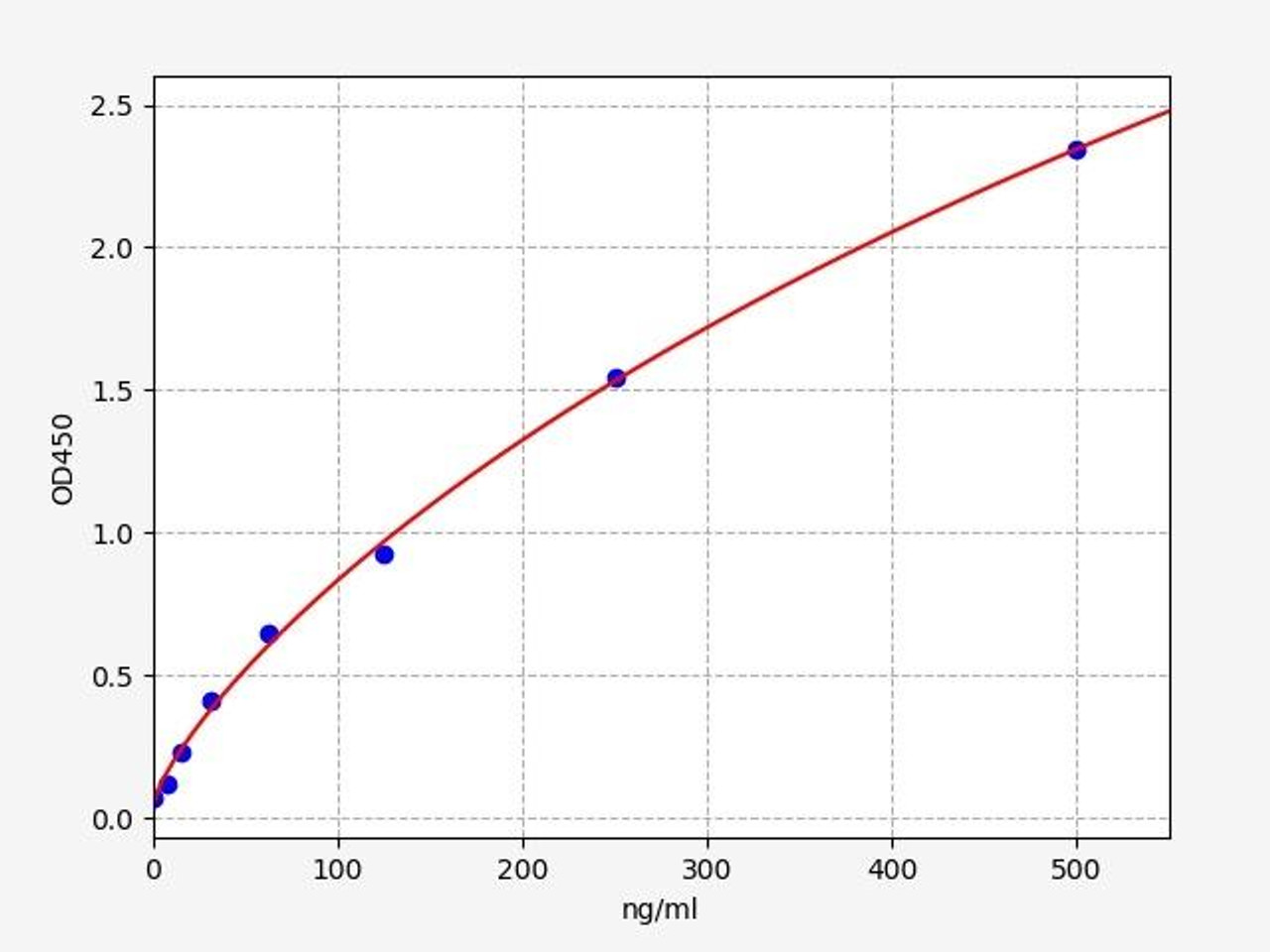
| Human Complement C3 / C3 ELISA Kit | |
|---|---|
| Sensitivity | 4.688ng/ml |
| Range | 7.813-500ng/ml |
| Reactivity | Human |
C3 (Complement C3) is a protein that, in humans, is encoded by the "C3" gene. Complement C3 encodes the third component of complement, a biochemical cascade of the immune system that helps to destroy invading pathogens.
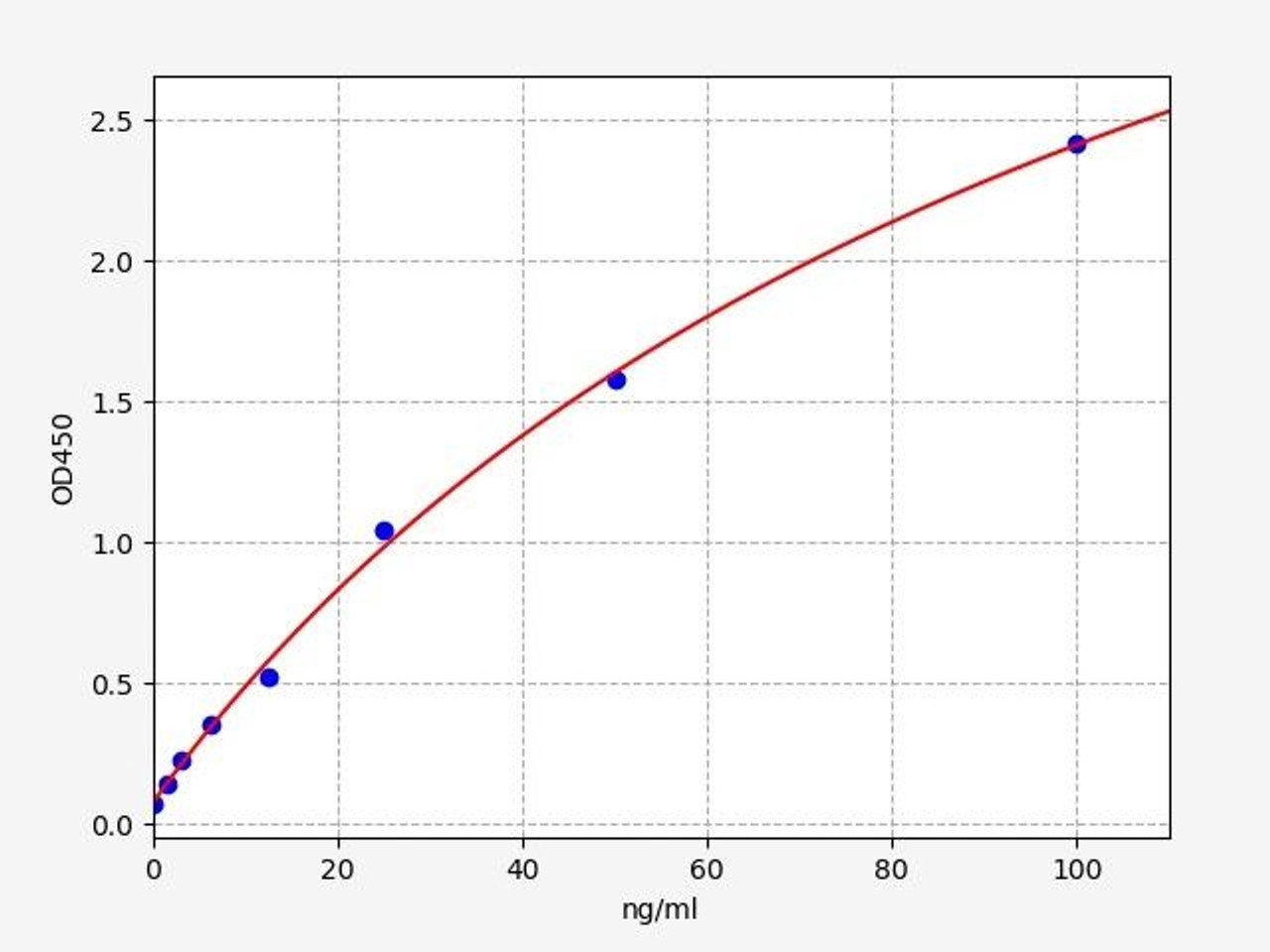
| Human Properdin / Complement Factor P ELISA Kit | |
|---|---|
| Sensitivity | 0.938ng/ml |
| Range | 1.563-100ng/ml |
| Reactivity | Human |
Properdin/Complement Factor P is a plasma glycoprotein that activates the alternative complement pathway of the body's innate immune system. Properdin/Complement Factor P binds to many microbial surfaces and apoptotic cells, keeping C3- and C5-convertase enzyme complexes in a feedback loop that ultimately produces the membrane attack complex and cell death.
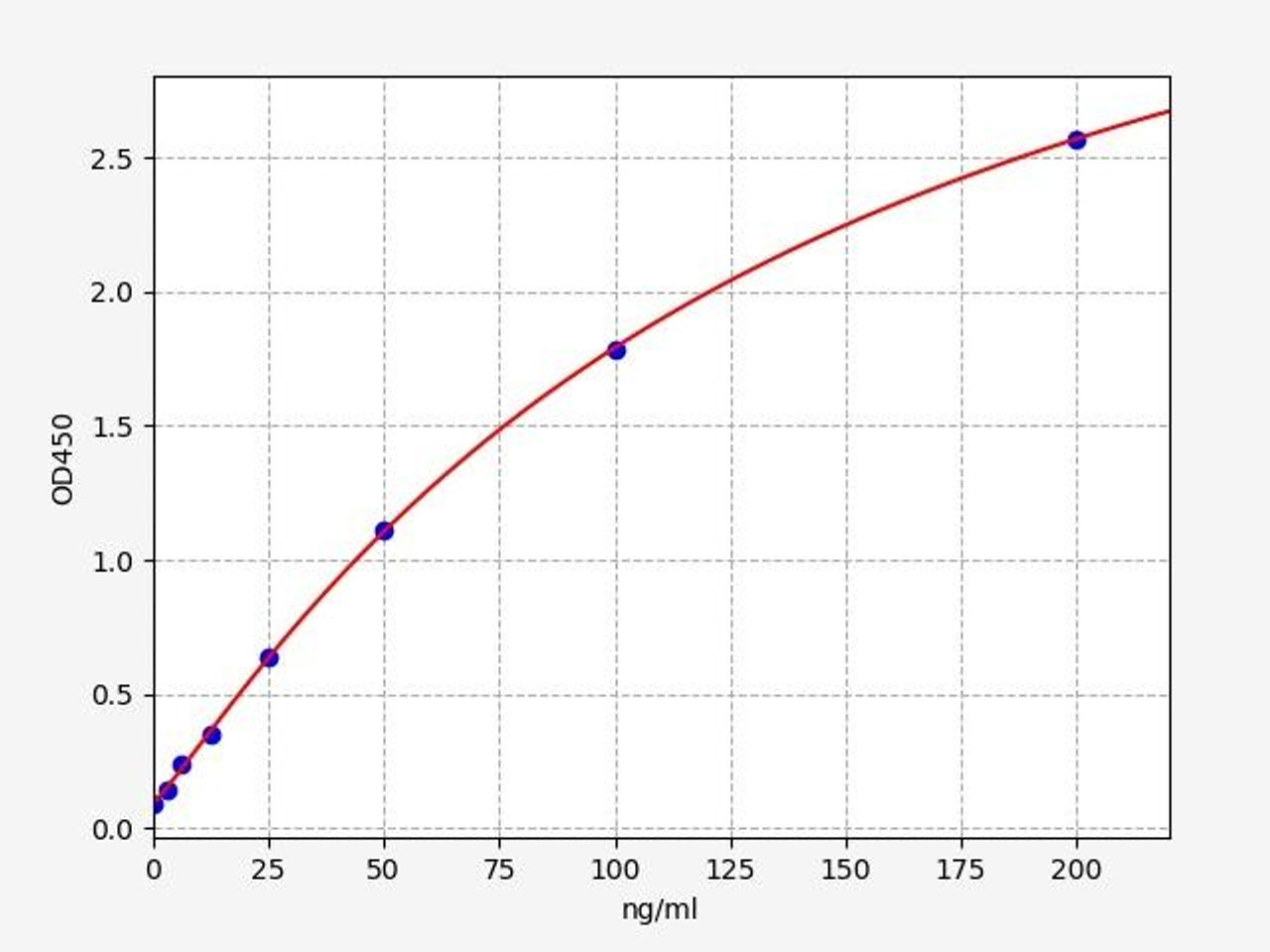
| Human CFB / Complement factor B ELISA Kit | |
|---|---|
| Sensitivity | 1.875ng/ml |
| Range | 3.125-200ng/ml |
| Reactivity | Human |
CFB (Complement Factor B) is a glycoprotein enzyme that is composed of two subunits, FB and FH. CFB is produced by the liver and is responsible for the degradation of heme, a component of hemoglobin. CFB deficiency can lead to serious health problems including anemia, jaundice, and neurologic problems.
The Complement System: Alternative Pathway and Beyond
Understanding the Alternative Pathway in the broader context of the complement system is crucial. It works synergistically with the other complement pathways, including the classical and lectin pathways, to mount a robust immune response. Each pathway has its unique triggers and modes of activation, ensuring the complement system can effectively combat a diverse range of pathogens.
Furthermore, dysregulation or deficiencies in the Alternative Pathway can have significant implications for human health. Disorders such as atypical hemolytic uremic syndrome (aHUS) and age-related macular degeneration (AMD) have been associated with aberrant complement activation through the Alternative Pathway. In aHUS, uncontrolled activation of the Alternative Pathway leads to the destruction of red blood cells and kidney damage. AMD, on the other hand, involves chronic inflammation and damage to the macula of the retina, with the Alternative Pathway playing a role in the development of the disease.
Research efforts are focused on understanding the underlying mechanisms of the Alternative Pathway and its dysregulation in various diseases. By gaining a deeper understanding of the triggers, regulators, and interactions of the Alternative Pathway, scientists aim to develop targeted therapies to modulate its activity. This could involve the development of specific inhibitors to halt excessive complement activation in diseases like aHUS or the identification of molecules that can enhance the Alternative Pathway in conditions where immune response is compromised.
In conclusion, the Alternative Pathway is a vital component of the complement system, working in synergy with the classical and lectin pathways to mount a robust immune response. Its unique mode of activation and its involvement in various diseases highlight its significance in maintaining immune homeostasis. Ongoing research holds the promise of uncovering new insights into the Alternative Pathway and its modulation, paving the way for potential therapeutic interventions in complement-related disorders.
Written by Pragna Krishnapur
Pragna Krishnapur completed her Bachelor degree in Biotechnology Engineering in Visvesvaraya Technological University before completing her Masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026




