Description
Recombinant Human Prolyl endopeptidase FAP Protein
The Recombinant Human Prolyl endopeptidase FAP Protein is a biologically active recombinant protein that plays a significant role in various cellular processes and signaling pathways in human biology. This protein is widely employed in immunological research, cell biology studies, protein-protein interaction analyses, and therapeutic development, providing researchers with a reliable tool for investigating Prolyl endopeptidase FAP function and its implications in health and disease.
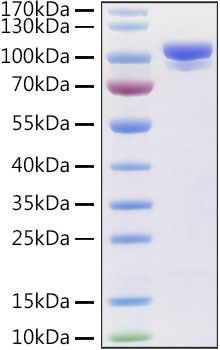
This product (SKU: RPCB0971) is produced using HEK293 cells and features a C-His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 85.87 kDa with an observed molecular weight of 95-100 kDa under denaturing conditions, achieving ≥ 95 % as determined by SDS-PAGE.. Functional bioactivity has been validated through rigorous quality control assays, confirming its suitability for demanding research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Human Prolyl endopeptidase FAP Protein |
| SKU: | RPCB0971 |
| Size: | 20 μg , 50 μg , 100 μg |
| Reactivity: | Human |
| Synonyms: | DPPIV, FAPA, FAPalpha, SIMP, FAP, DPPIV, prolyl endopeptidase FAP, FAPA, FAPalpha, SIMP, Fibroblast activation protein alpha, FAP |
| Tag: | C-His |
| Expression Host: | HEK293 cells |
| Calculated MW: | 85.87 kDa |
| Observed MW: | 95-100 kDa |
| Gene ID: | 2191 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Human Prolyl endopeptidase FAP Protein (RP01348LQ), tested reactivity in HEK293 cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 0.1 EU/μg of the protein by LAL method. |
| Purity: | ≥ 95 % as determined by SDS-PAGE. |
| Formulation: | Supplied as a 0.22 μm filtered solution in PBS, pH 7.4. |
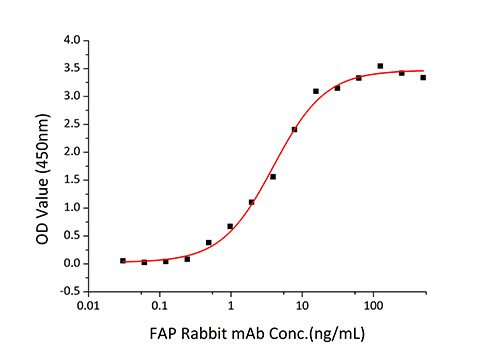
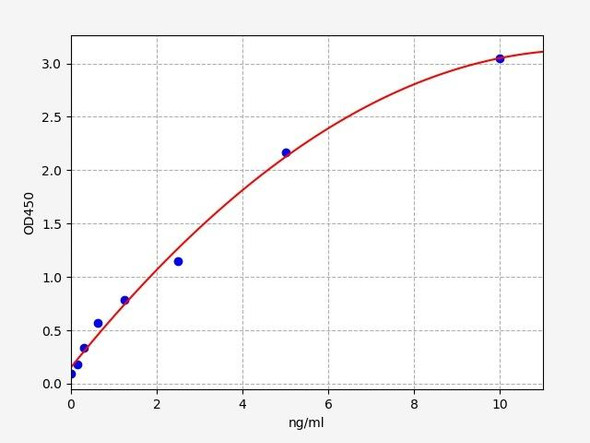
| Bio-Activity: |
|
| Storage: | Store at -70℃. This product is stable at ≤ -70℃ for up to 1 year from the date of receipt. For optimal storage, aliquot into smaller quantities after centrifugation and store at recommended temperature. Avoid repeated freeze-thaw cycles. |
FAP (also known as seprase) is a Type II transmembrane serine protease,which belongs to thepeptidase S9B family. Seprase / FAP is found in cell surface lamellipodia, invadopodia and on shed vesicles. Seprase / FAP appears to act as a proteolytically active 17-kDa dimer, consisting of two 97-kDa subunits. It is a member of the group type II integral serine proteases, which includes dipeptidyl peptidase IV ( DPPIV / CD26 ) and related type II transmembrane prolyl serine peptidases, which exert their mechanisms of action on the cell surface. Seprase / FAP colocalized with DPP4 in invadopodia and lamellipodia of migratory activated endothelial cells in collagenous matrix. Seprase / FAP colocalized with DPP4 on endothelial cells of capillary-like microvessels but not large vessels within invasive breast ductal carcinoma. DPP4 and seprase exhibit multiple functions due to their abilities to form complexes with each other and to interact with other membrane-associated molecules. In association with DPP4, Seprase / FAP is involved in the pericellular proteolysis of the extracellular matrix (ECM), the migration and invasion of endothelial cells into the ECM. Seprase / FAP has a dual function in tumour progression. The proteolytic activity of Seprase has been shown to promote cell invasiveness towards the ECM and also to support tumour growth and proliferation. Seprase / FAP may have a role in tissue remodeling during development and wound healing, and may contribute to invasiveness in malignant cancers.