Description
Recombinant Human Siglec-6/CD327 Protein
The Recombinant Human Siglec-6/CD327 Protein is a biologically active recombinant protein that plays a significant role in various cellular processes and signaling pathways in human biology. This protein is widely employed in immunological research, cell biology studies, protein-protein interaction analyses, and therapeutic development, providing researchers with a reliable tool for investigating Siglec-6/CD327 function and its implications in health and disease.
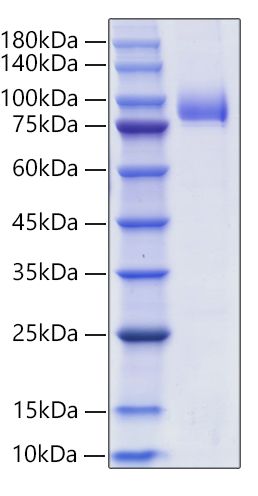
This product (SKU: RPCB1591) is produced using HEK293 cells and features a C-hFc&6*His tag for convenient detection and purification. The protein exhibits a calculated molecular weight of 58.31 kDa with an observed molecular weight of 75-100 kDa under denaturing conditions, achieving ≥ 95% as determined by SDS-PAGE., ensuring exceptional quality and consistency for research applications.
Key Features
| High Purity by Affinity Chromatography | |
| Mammalian & Bacterial Expression Systems | |
| High lot-to-lot consistency via strict QC |
| Product Name: | Recombinant Human Siglec-6/CD327 Protein |
| SKU: | RPCB1591 |
| Size: | 20 μg |
| Reactivity: | Human |
| Synonyms: | SIGLEC6, CD33L, CD33L1, OBBP1, Sialic acid-binding Ig-like lectin 6, Siglec-6, CD33 antigen-like 1, CDw327, Obesity-binding protein 1, OB-BP1, CD327 |
| Tag: | C-hFc&6*His |
| Expression Host: | HEK293 cells |
| Calculated MW: | 58.31 kDa |
| Observed MW: | 75-100 kDa |
| Gene ID: | 946 |
| Protein Description: | High quality, high purity and low endotoxin recombinant Recombinant Human Siglec-6/CD327 Protein (RPCB1591), tested reactivity in HEK293 cells and has been validated in SDS-PAGE.100% guaranteed. |
| Endotoxin: | < 0.1 EU/μg of the protein by LAL method. |
| Purity: | ≥ 95% as determined by SDS-PAGE. |
| Formulation: | Lyophilized from a 0.22 μm filtered solution of PBS, pH 7.4. |
| Reconstitution: | Centrifuge the vial before opening. Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Avoid vortex or vigorously pipetting the protein. For long term storage, it is recommended to add a carrier protein or stablizer (e.g. 0.1% BSA, 5% HSA, 10% FBS or 5% Trehalose), and aliquot the reconstituted protein solution to minimize free-thaw cycles. |
| Storage: | Store at -20℃.Store the lyophilized protein at -20℃ to -80 ℃ up to 1 year from the date of receipt. After reconstitution, the protein solution is stable at -20℃ for 3 months, at 2-8℃ for up to 1 week. |
Siglecs (sialic acid binding Ig-like lectins) are I-type (Ig-type) lectins that belong to the Ig superfamily. They are characterized by an N-terminal Ig-like V-type domain which mediates sialic acid binding, followed by varying numbers of Ig-like C2-type domains. Eleven human Siglecs (Siglec-1 through 11) have been cloned and characterized. Within these eleven, there are at least two groups, one of which is termed the CD33-related group. CD33-related Siglecs include CD33/Siglec-3 and Siglec-5 through 11. To date, no Siglec has been shown to recognize any cell surface ligand other than sialic acid. This suggests that interactions with glycans containing this carbohydrate are important in mediating the biological functions of Siglecs. The cDNA of human Siglec-6 (also known as OB-BP1 and CD33L), encodes a putative 442 amino acid (aa) protein that contains a 15 aa signal peptide, a 321 aa extracellular region, a 21 aa transmembrane region (TM), and an 85 aa cytoplasmic tail. The extracellular region contains one N-terminal V-type Ig-like domain followed by two Ig-like C2-type domains. The cytoplasmic domain has one immunoreceptor tyrosine-based inhibition motif (ITIM). At least three additional isoforms exist, all of which encode an additional 11 aa’s at the N-terminus, likely due to the utilization of an alternate start site. Two of the three isoforms also show splicing. One isoform shows a 16 aa in-frame deletion in the second C2-like domain, while the other shows a deletion of the TM and cytoplasmic region, thus potentially generating a soluble form.