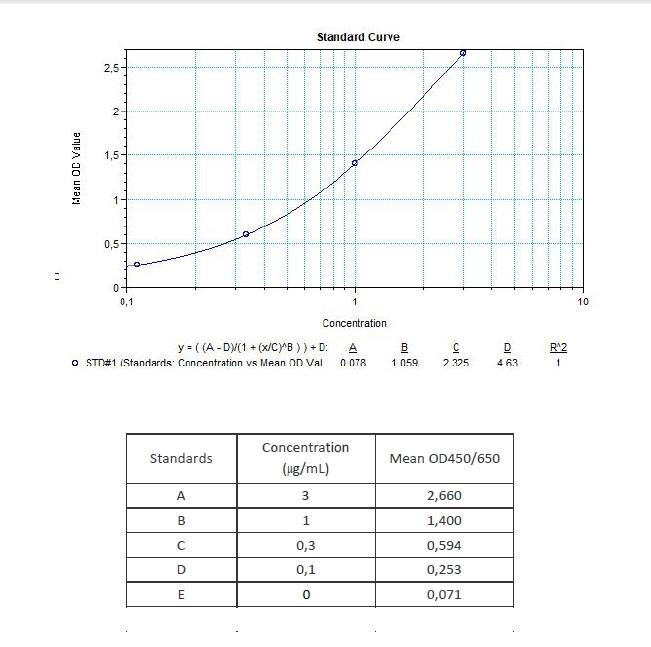
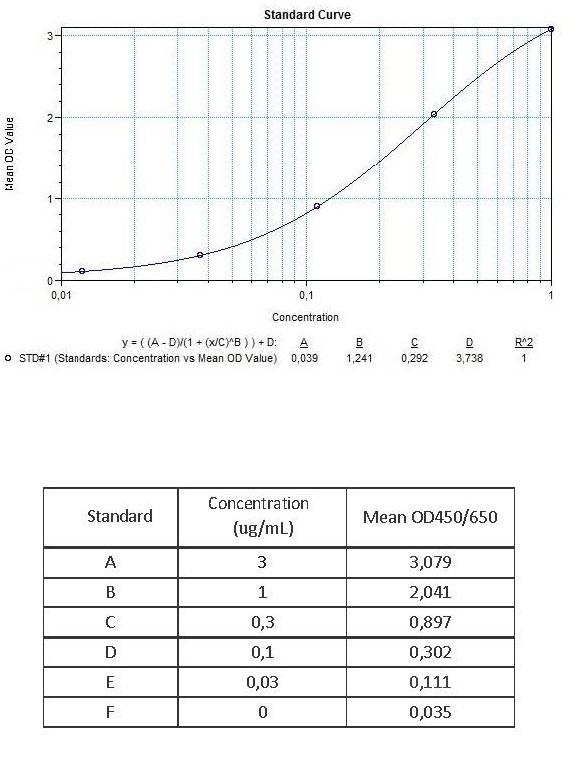
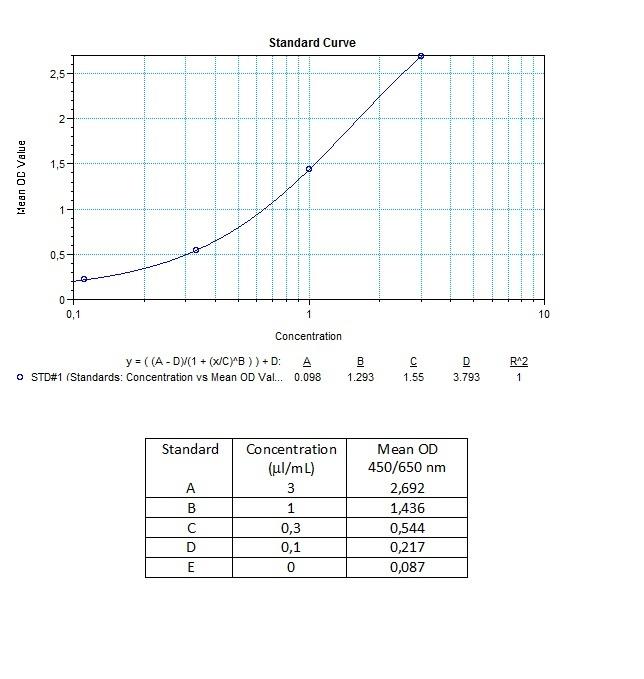
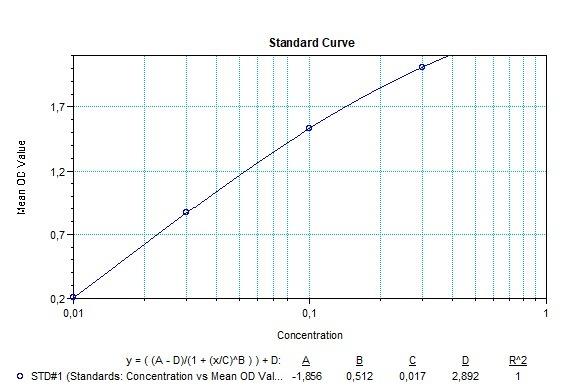
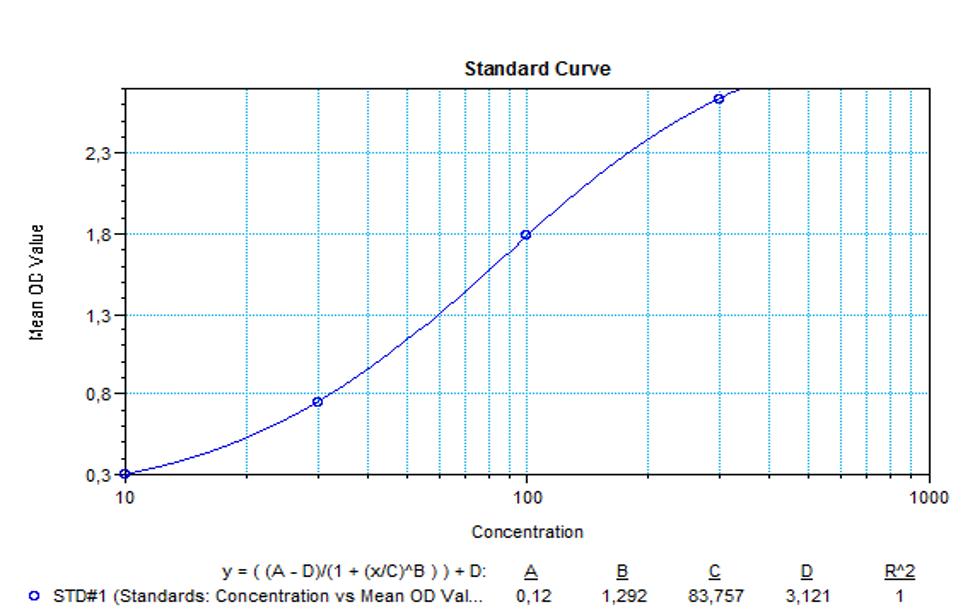
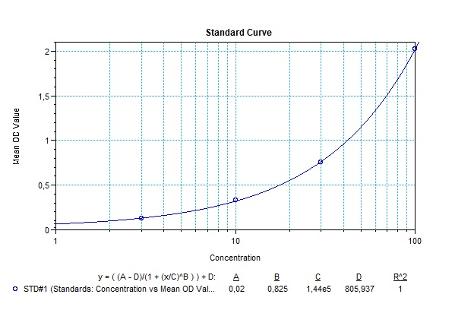
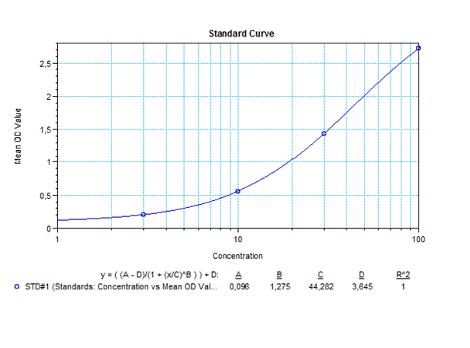
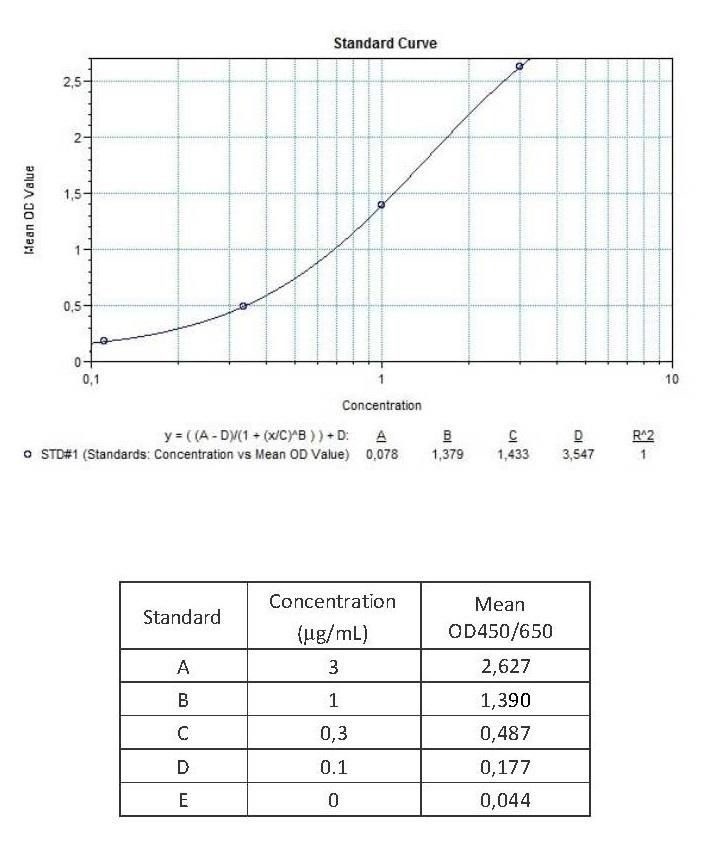
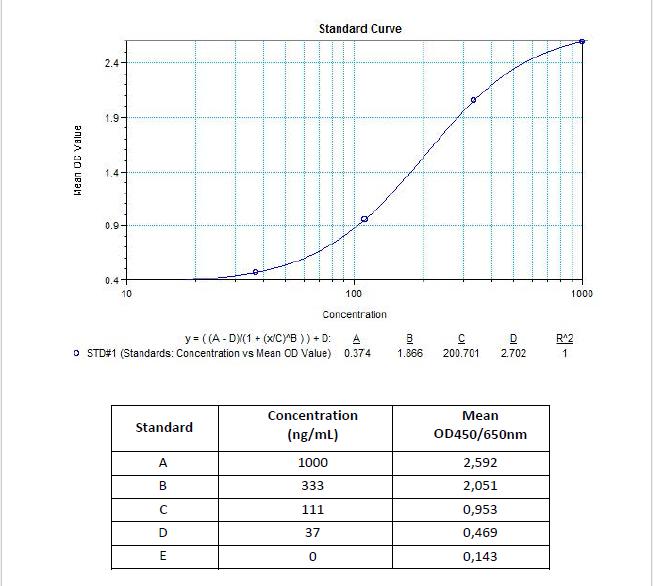
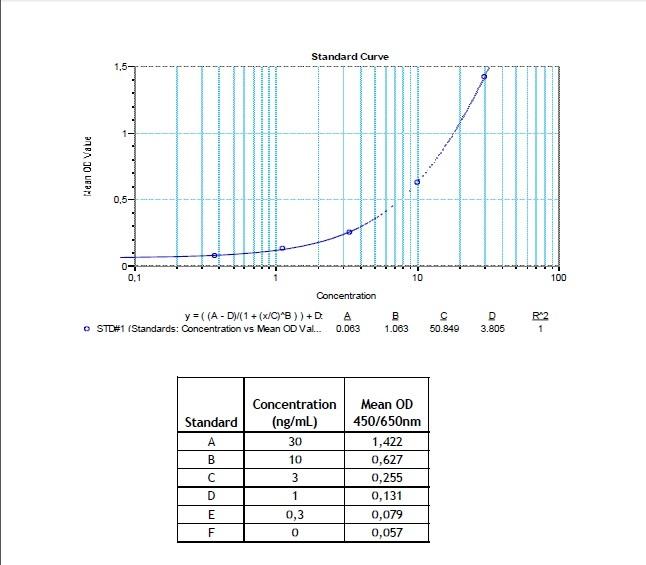
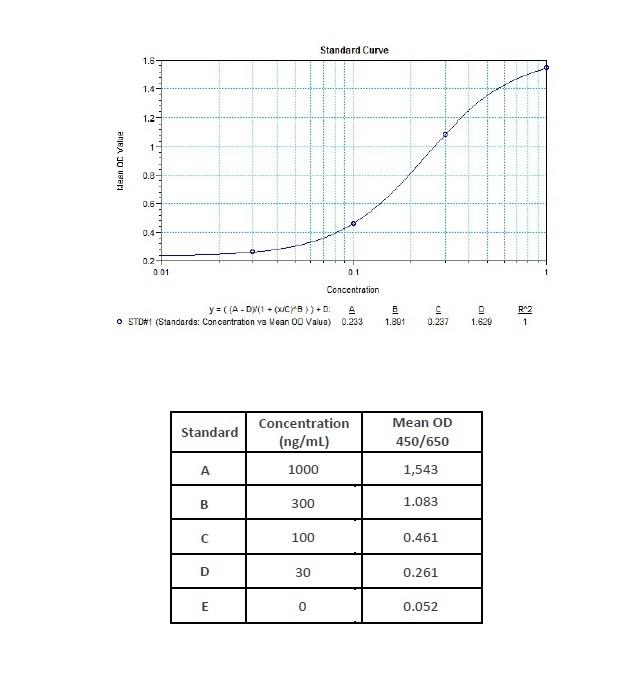
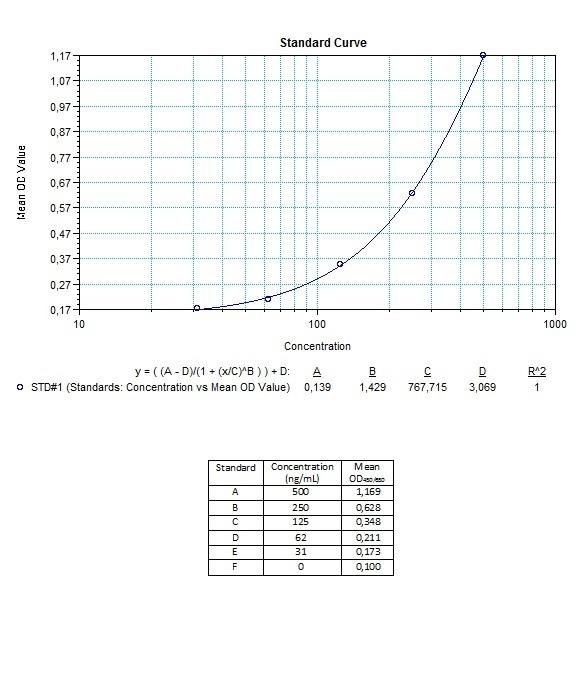
Therapeutic Antibody & Biosimilar ELISA Kits
Therapeutic Antibody & Biosimilar ELISA Kits
Highly Sensitive Quantitative Kits
Designed for pharmacokinetic (PK), immunogenicity, and ADA studies, our kits support accurate analysis in preclinical and clinical samples across various species

Why Choose ELISA for Drug Monitoring?
Key Features

Our TDM & ADA ELISA Range
Multiple options and formats

Therapeutic mAb ELISA

Biosimilar
ELISA

ADA
ELISA

Custom
ELISA
Popular Targets
Testimonials & Partners
Supporting Scientists Worldwide


Citations
Product | Citation |
|---|---|
Chemical Communications Sensi et al. | |
Cancer Chemotherapy and Pharmacology Gandhi et al. | |
cGMP & ISO Certification
High-quality products


Quality is at the core of everything we do.
Manufactured in state-of-the-art facilities, our products meet the highest standards, including current Good Manufacturing Practicies) cGMP, ISO 9001:2015, and ISO 13485:2016 certifications. These ensure consistent quality, customer-focused innovation, and reliable performance in every batch.
ELISA Manufacture Pipeline

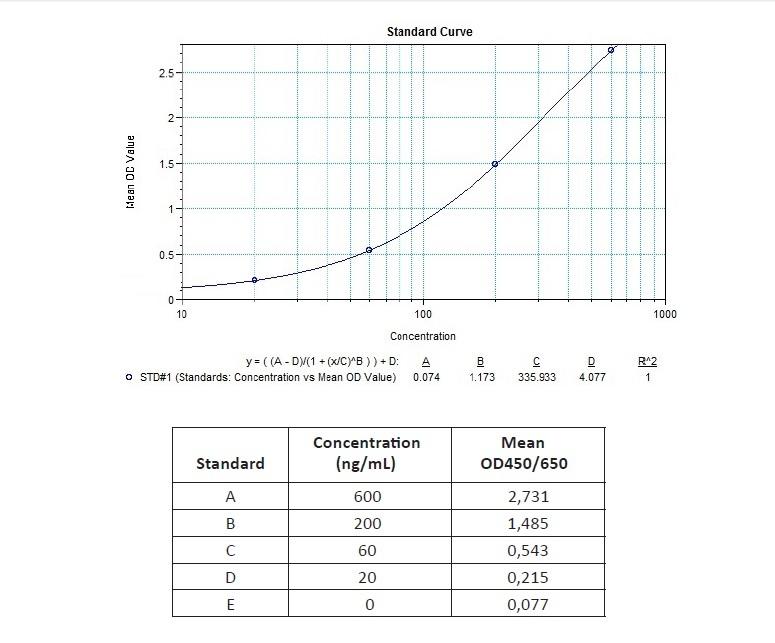
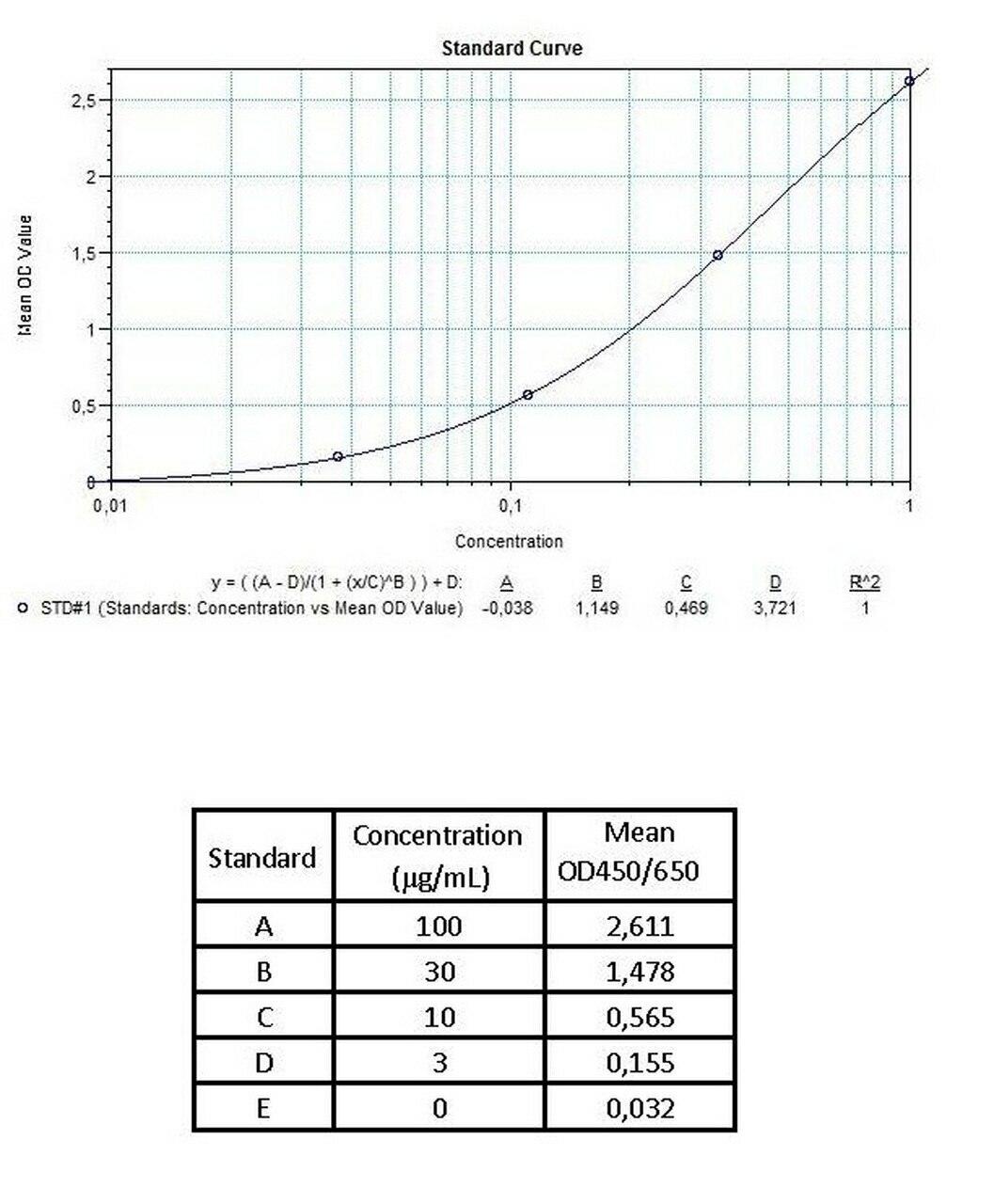
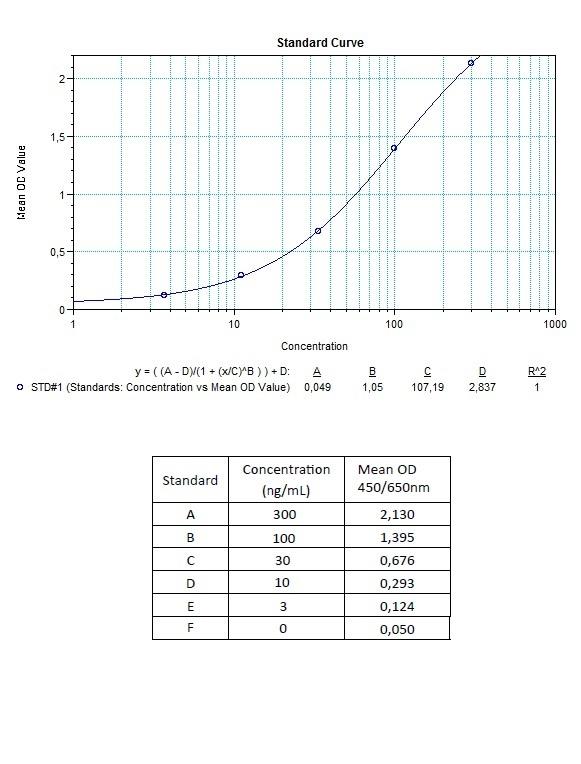
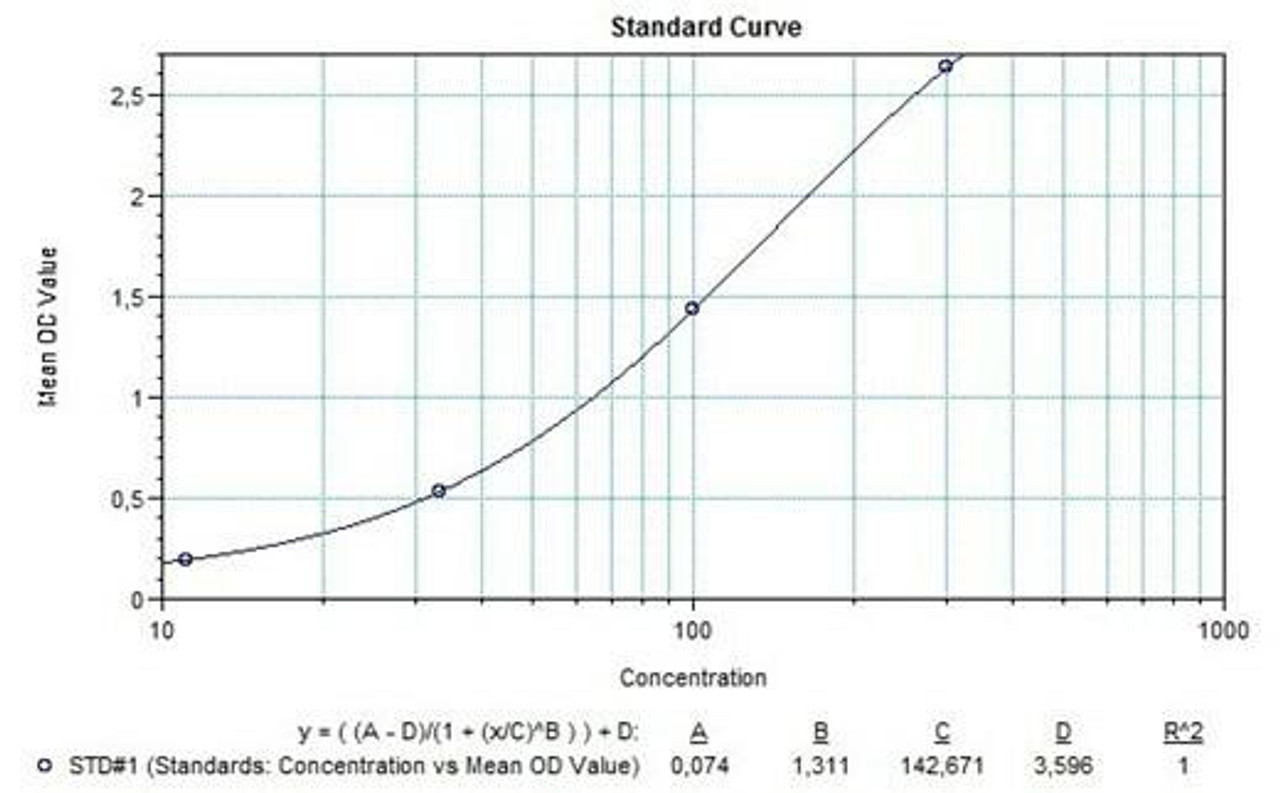
Detection Principle & Protocol
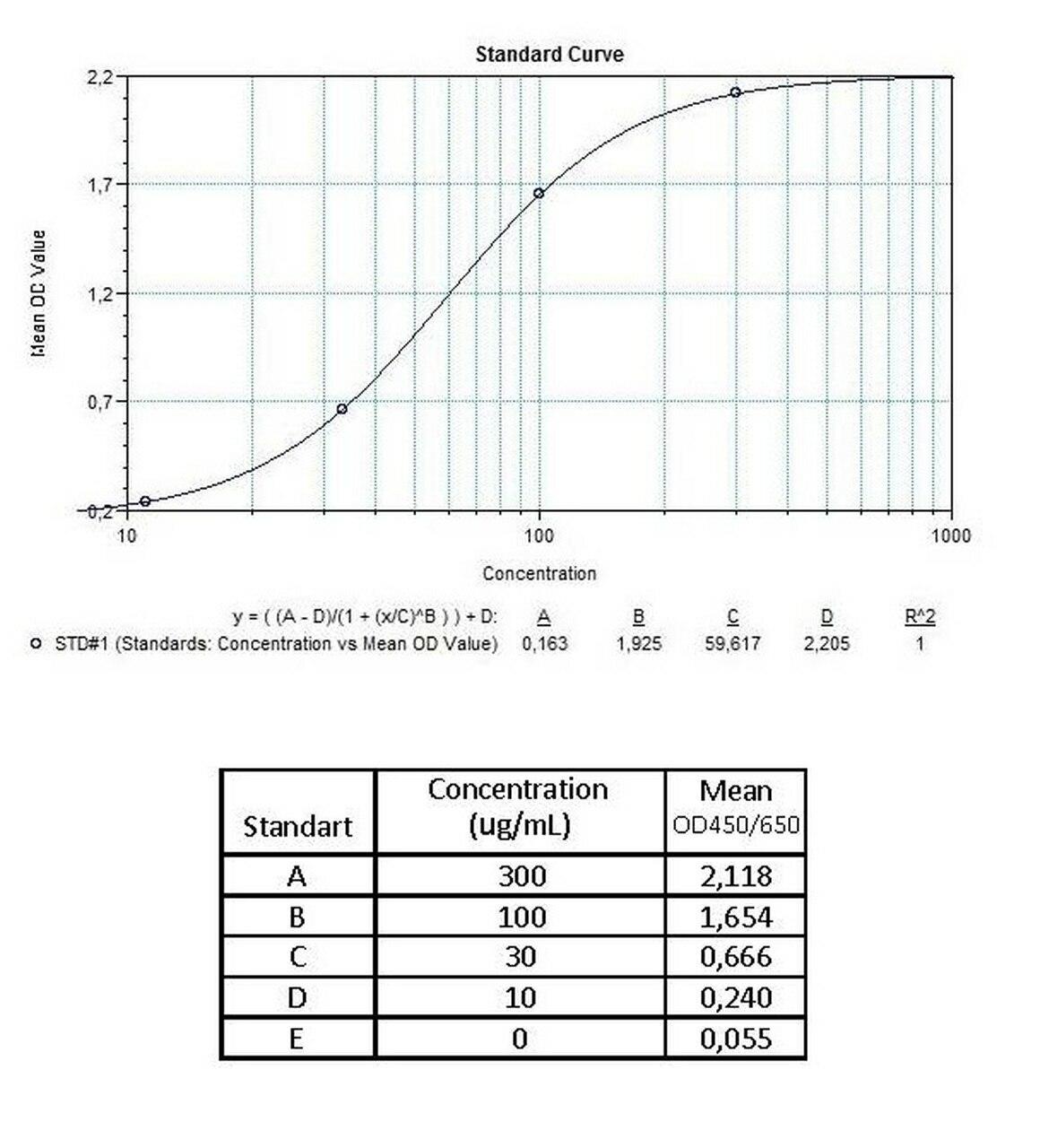
An ELISA protocol involves coating a microplate with a specific capture antibody, adding the sample containing the target analyte, and incubating to allow binding. After washing to remove unbound substances, a detection antibody (linked to an enzyme) is added, followed by a substrate solution that produces a measurable color change. The optical density (OD) is read using a spectrophotometer, with absorbance values correlating to analyte concentration.

What are Therapeutic Antibodies & ADAs?
- Therapeutic mAbs | Designed to bind specific targets and modulate the immune responses or block disease-related pathways.
- Biosimilars | Highly similar versions of approved therapeutic mAbs, while offering a more cost-effective alternative.
- Anti-Drug Antibodies (ADAs) | Immune-generated antibodies that can bind or neutralize therapeutic mAbs, reducing effectiveness.

Why is drug monitoring important ?

- Optimized Dosing | Ensures therapeutic drug concentrations remain within the optimal range for maximum efficacy.
- Reduced Immunogenicity | Prevents overdosing-related side effects and identifies ADA formation that may reduce drug effectiveness.
- Personalized Treatment | Enables dose adjustments based on individual patient responses, improving long-term outcomes
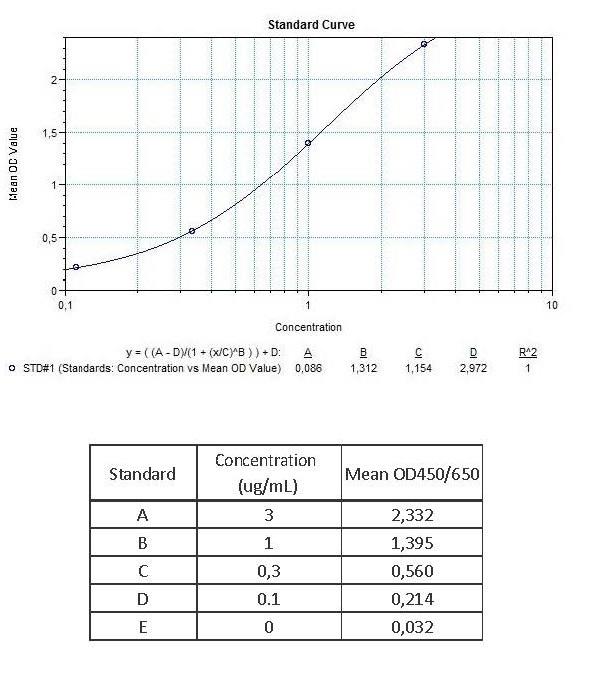
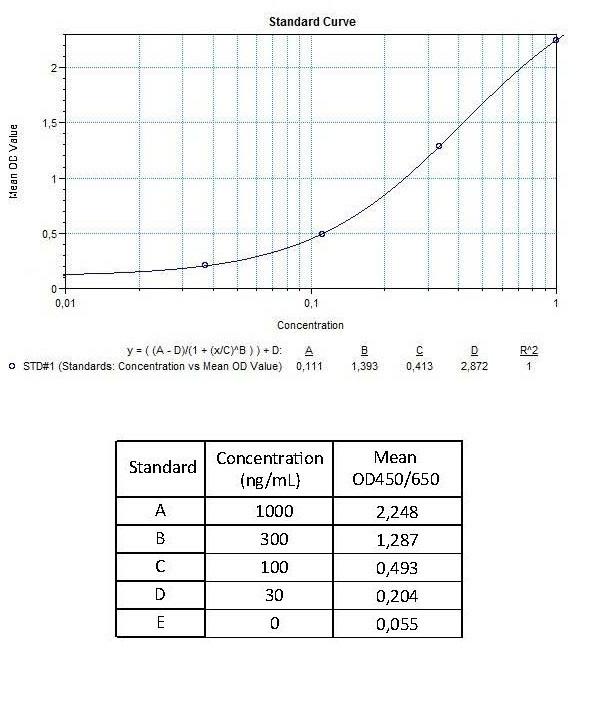
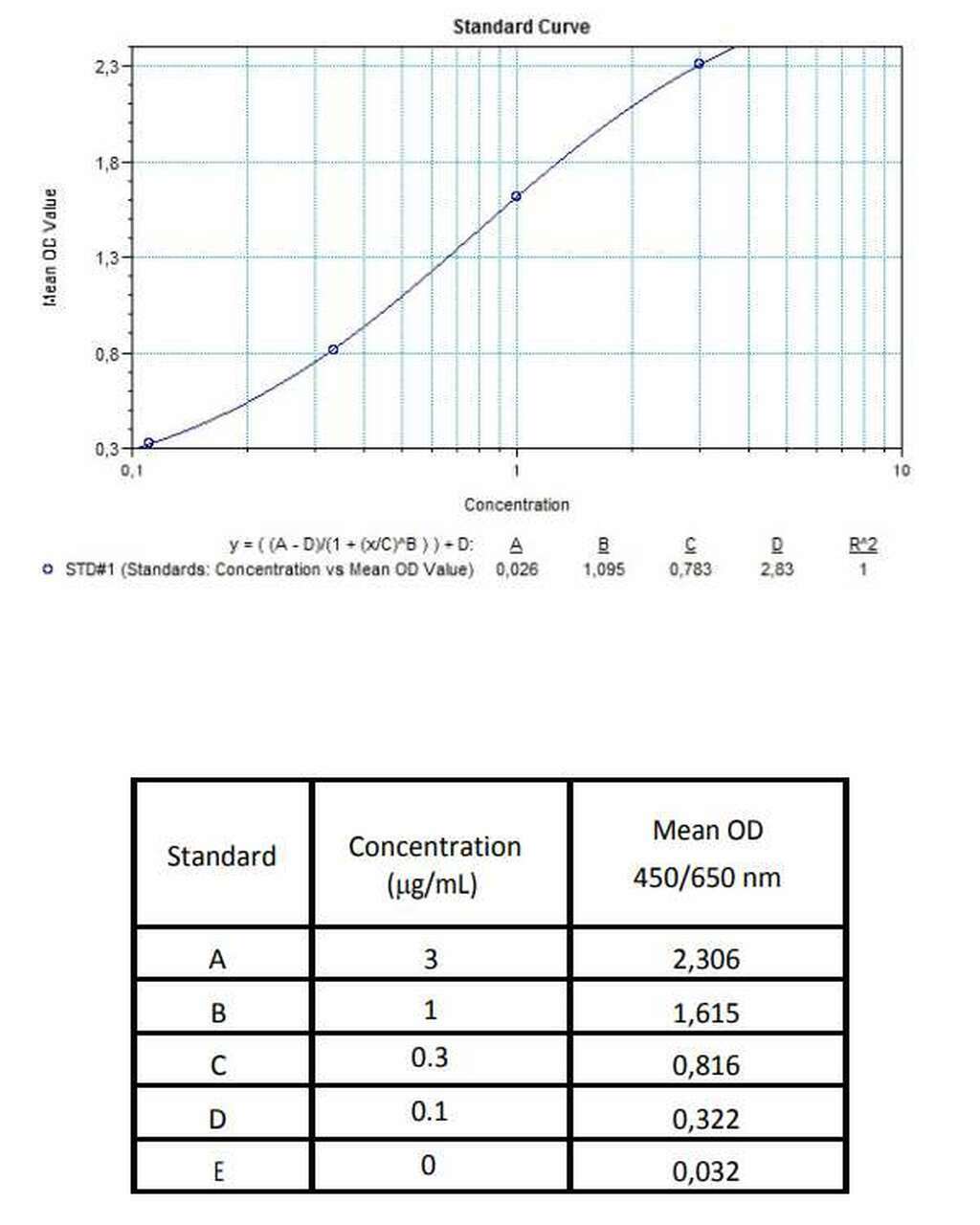
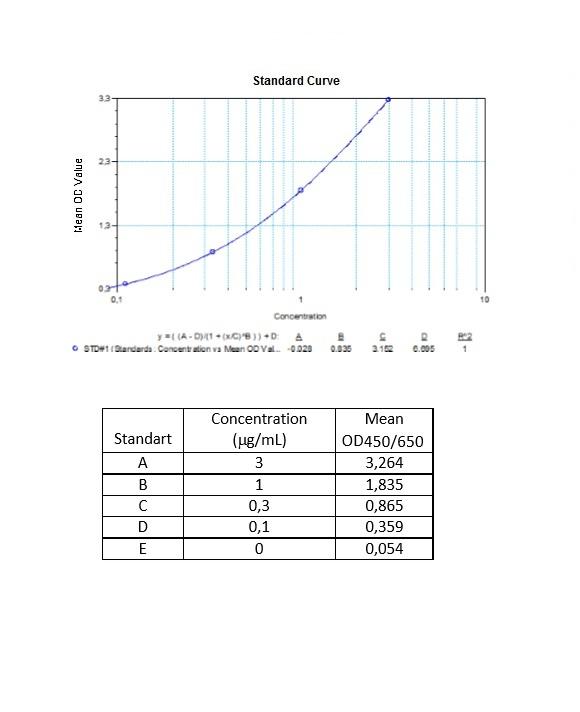
Applications & Data
Therapeutic mAb & Biosimilar ELISA
- Quality Control | ELISA can be used for QC during the development and manufacturing of antibodies
- Biosimilarity assessment | Test similarity between biosimilar and reference product
- PK studies | Assess how mAbs move through the body and help to determine optimal dosing.
- Clinical Development | Monitor the presence of therapeutic & biosimilar mAb in patient samples

Anti-Drug Antibody (ADA) ELISA
- Immunogenicity Assessment | High levels of ADAs may neutralize the therapeutic effects of the drug, rendering it less effective.
- Response Variability | Identify differences in patients response by measuring ADA levels
- Biosimilarity Evaluation | Helps to assess the immunogenicity profile of biosimilars to reference product
- Free vs. Total ADA | Acid dissociation technology breaks antibody complex making ADA detectable
Meet Some of the Genie Team!
At Assay Genie, we're here to help every step of the way. Whether you need guidance choosing the right product, updates on your order, or expert support after your purchase, we've got you covered.
Frequently asked questions
Research related ELISA Kits
Designed to meet your research needs
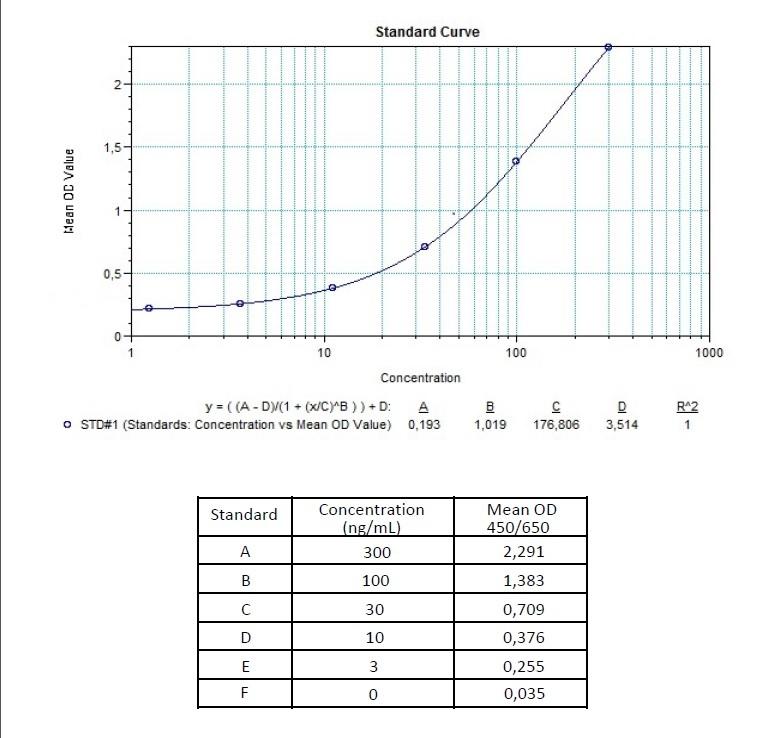
| Anti-Ramucirumab (Cyramza®) ADA | |
|---|---|
| Product Code | HUMB00023 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |
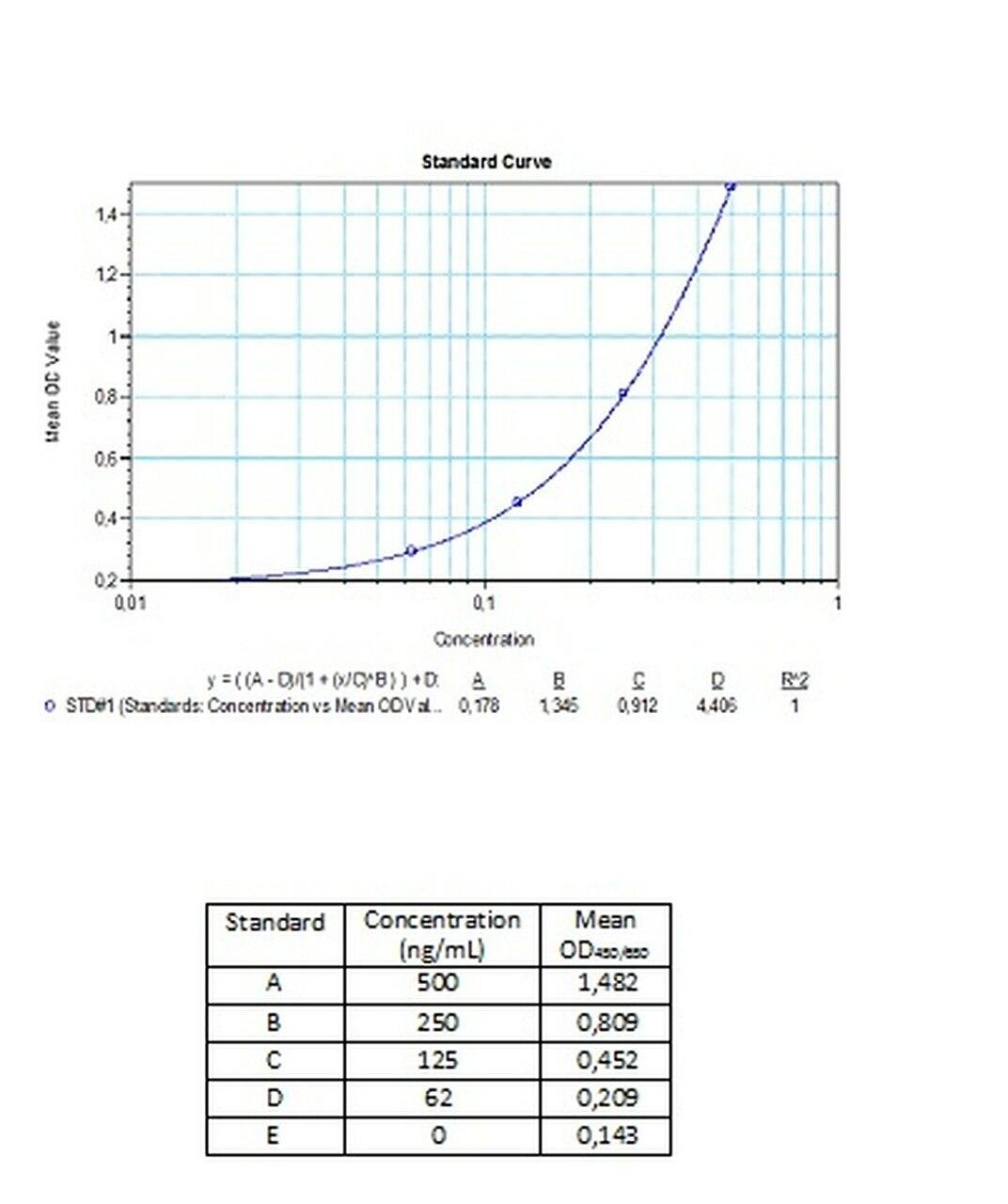
| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |

| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |
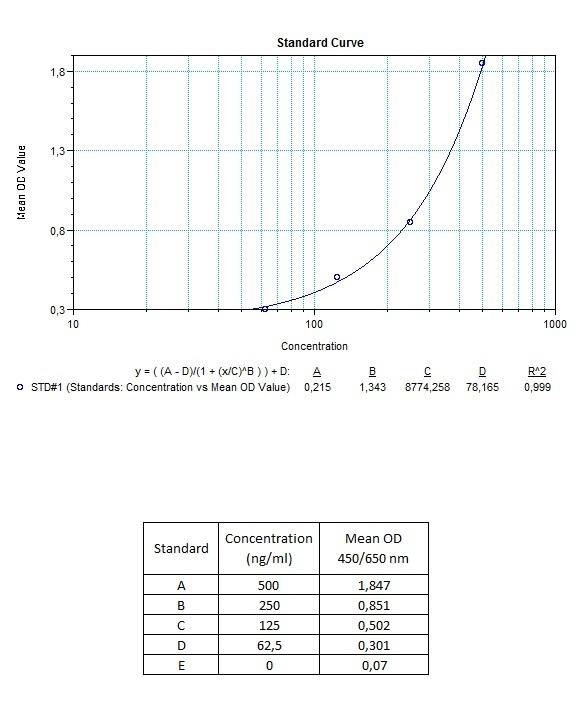
| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00028 |
| ELISA Type | Antibody screening - Qualitative |
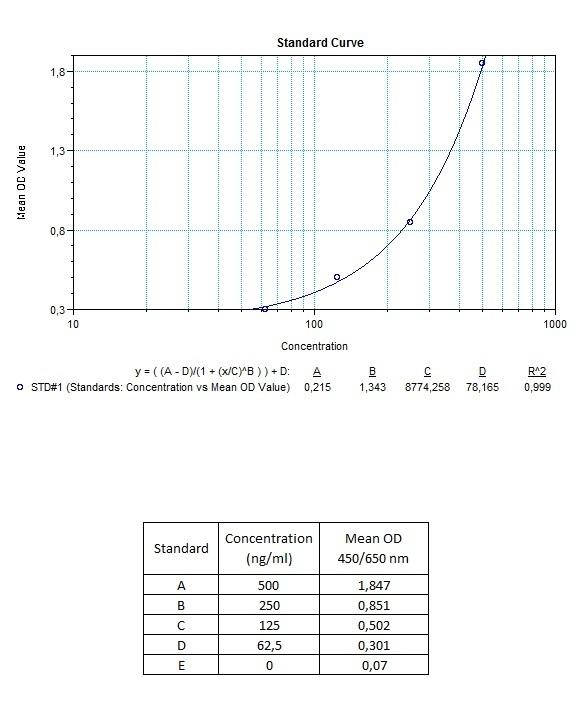
| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00029 |
| ELISA Type | Antibody screening - Quantitative |
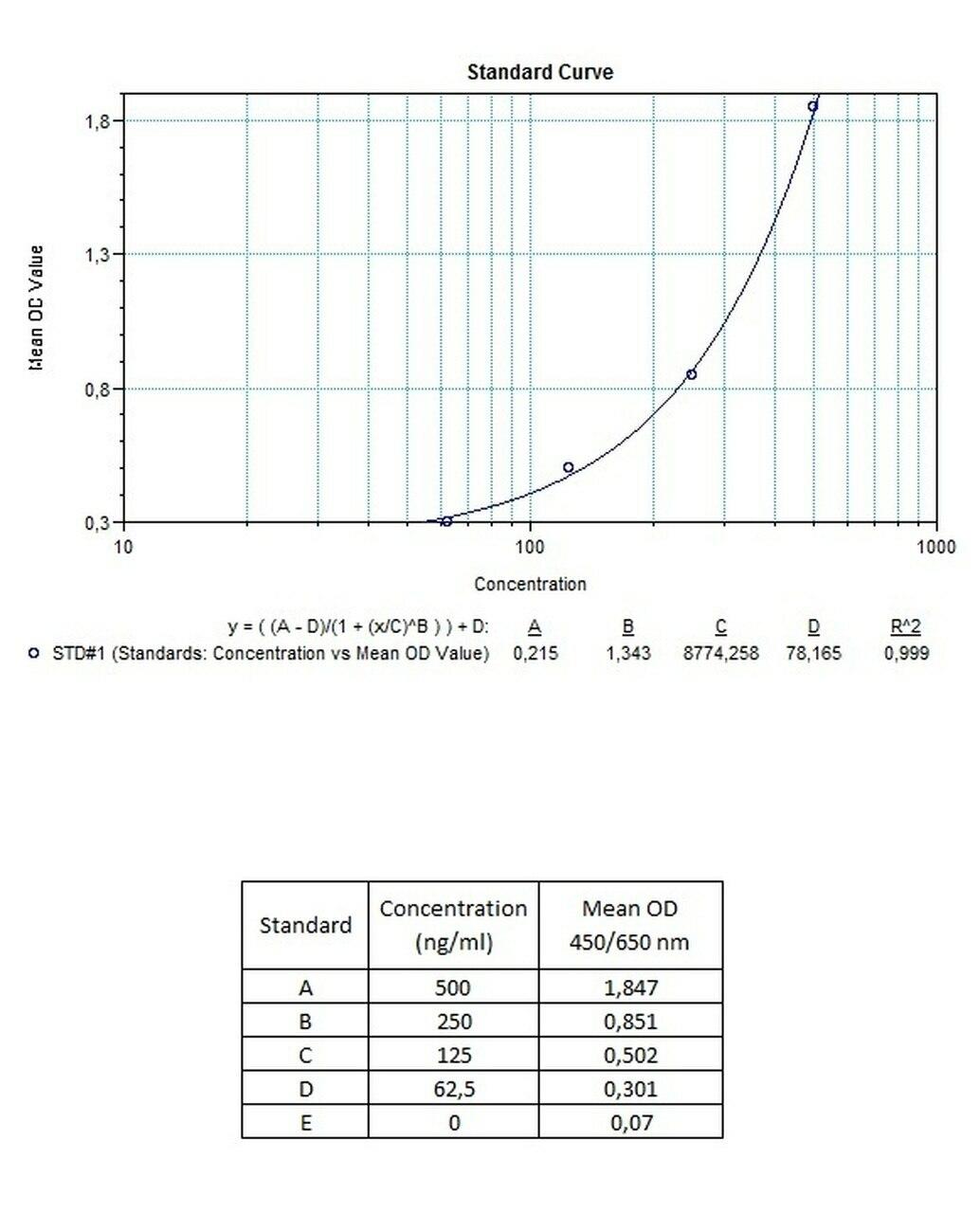
| Anti-Trastuzumab (Herceptin®, Herclon®) ADA | |
|---|---|
| Product Code | HUMB00031 |
| ELISA Type | Antibody screening - Qualitative |
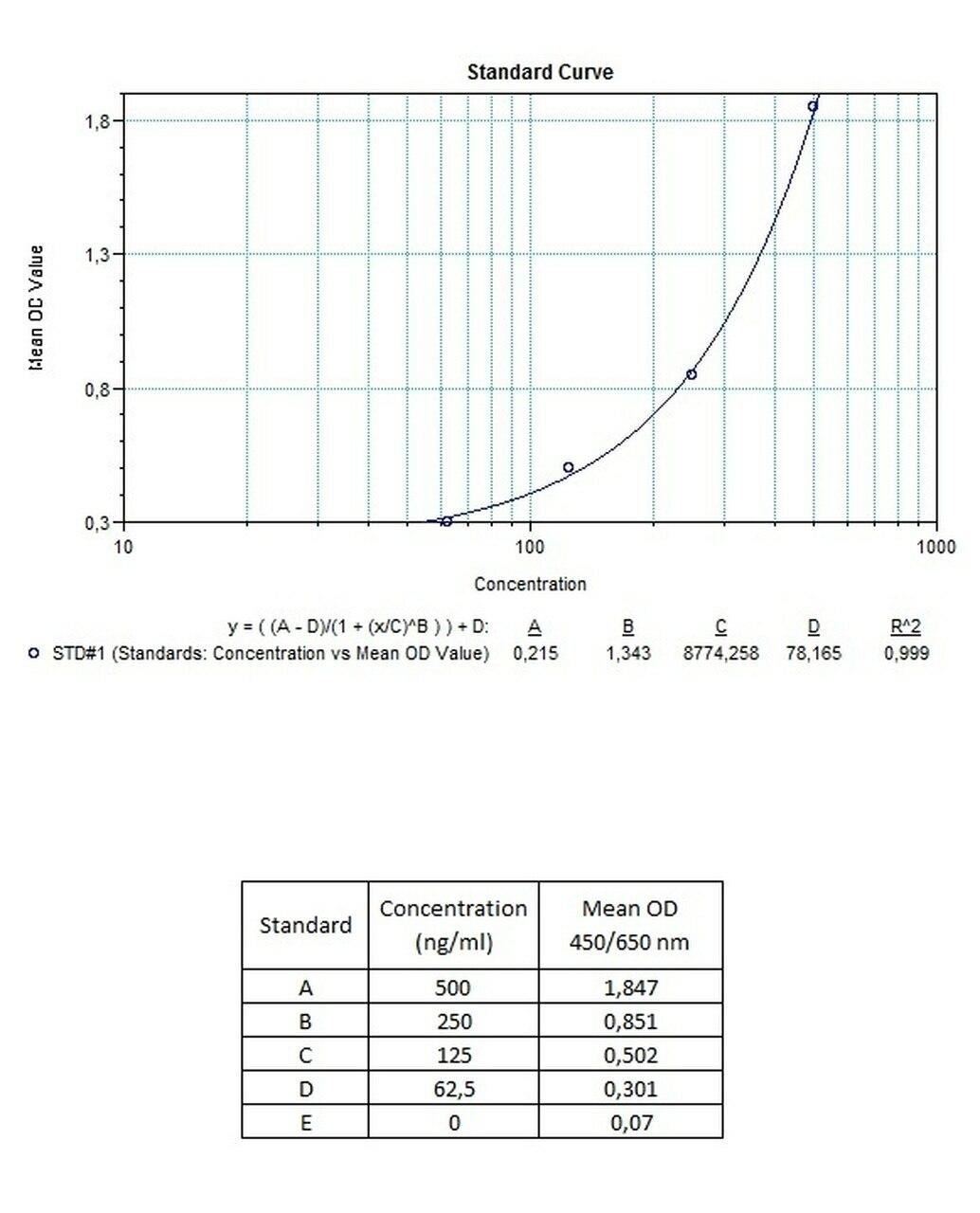
| Anti-Trastuzumab (Herceptin®, Herclon®) ADA | |
|---|---|
| Product Code | HUMB00032 |
| ELISA Type | Antibody screening - Quantitative |
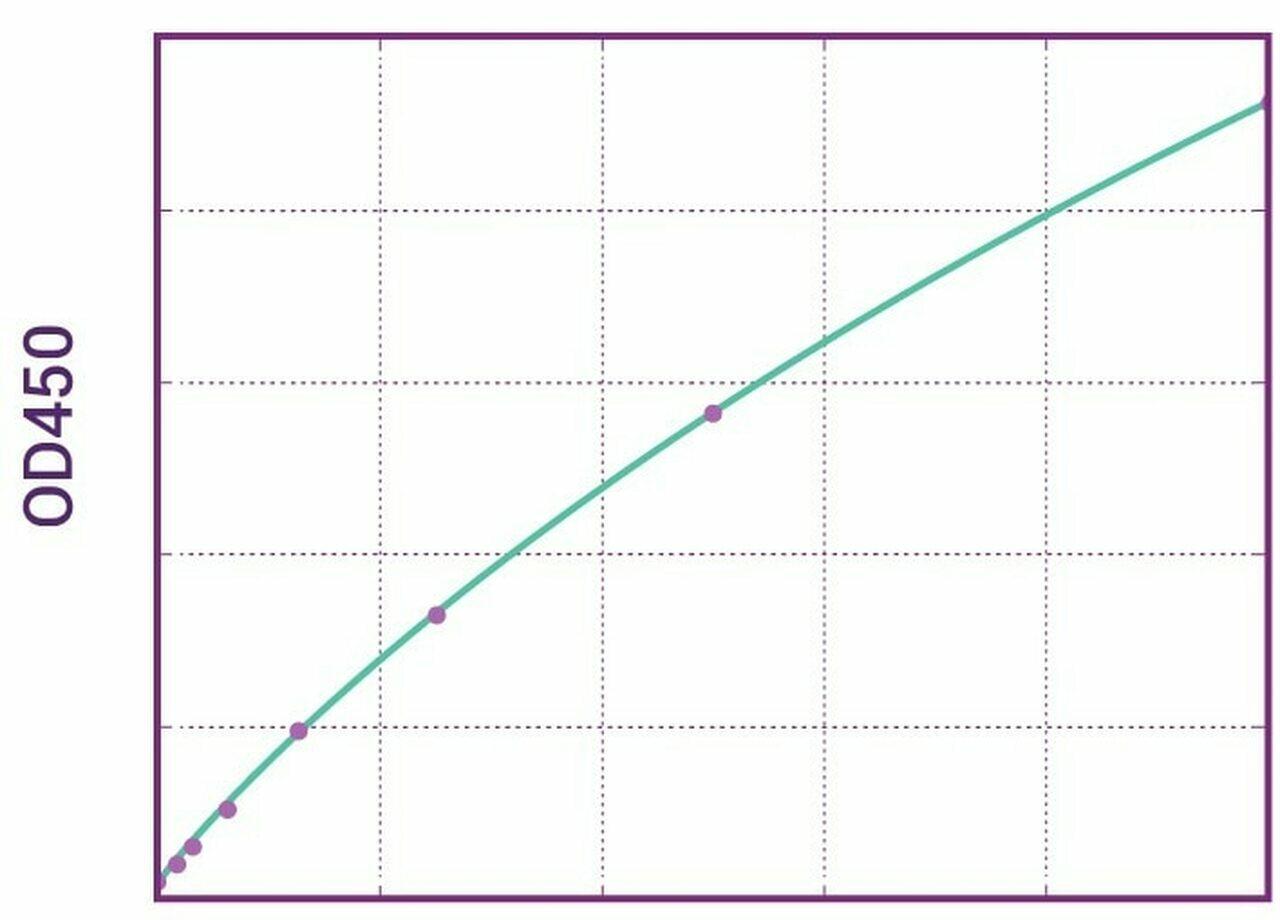
| Anti-Cetuximab (Erbitux®) ADA | |
|---|---|
| Product Code | HUMB00034 |
| ELISA Type | Antibody screening - Qualitative |
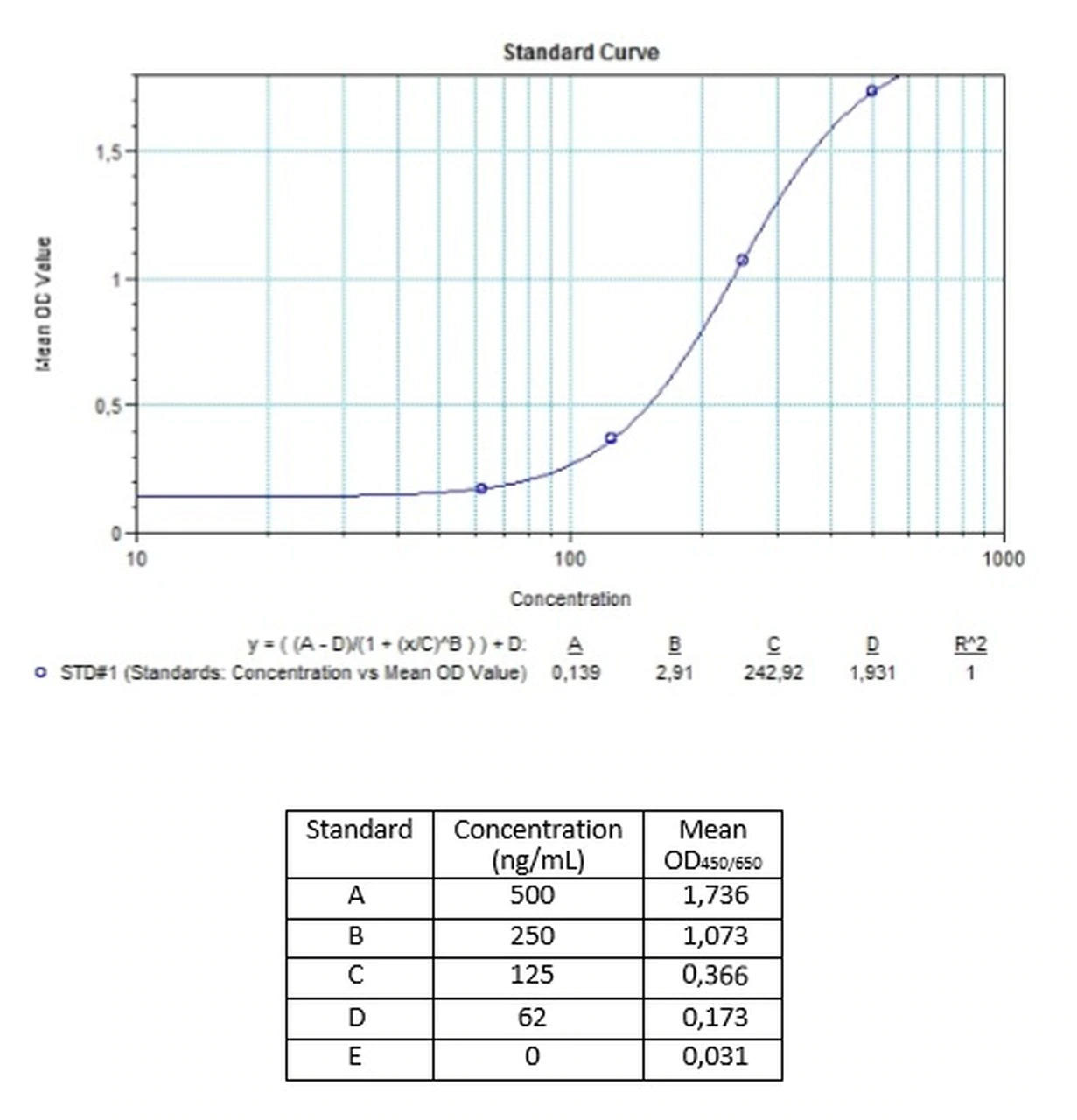
| Anti-Cetuximab (Erbitux®) ADA | |
|---|---|
| Product Code | HUMB00035 |
| ELISA Type | Antibody screening - Quantitative |
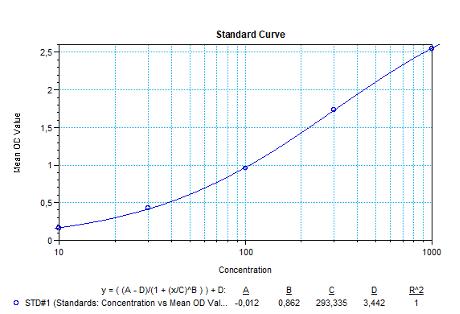
| Daratumumab (Darzalex®) ADA | |
|---|---|
| Product Code | HUMB00063 |
| ELISA Type | Antibody screening - Qualitative |
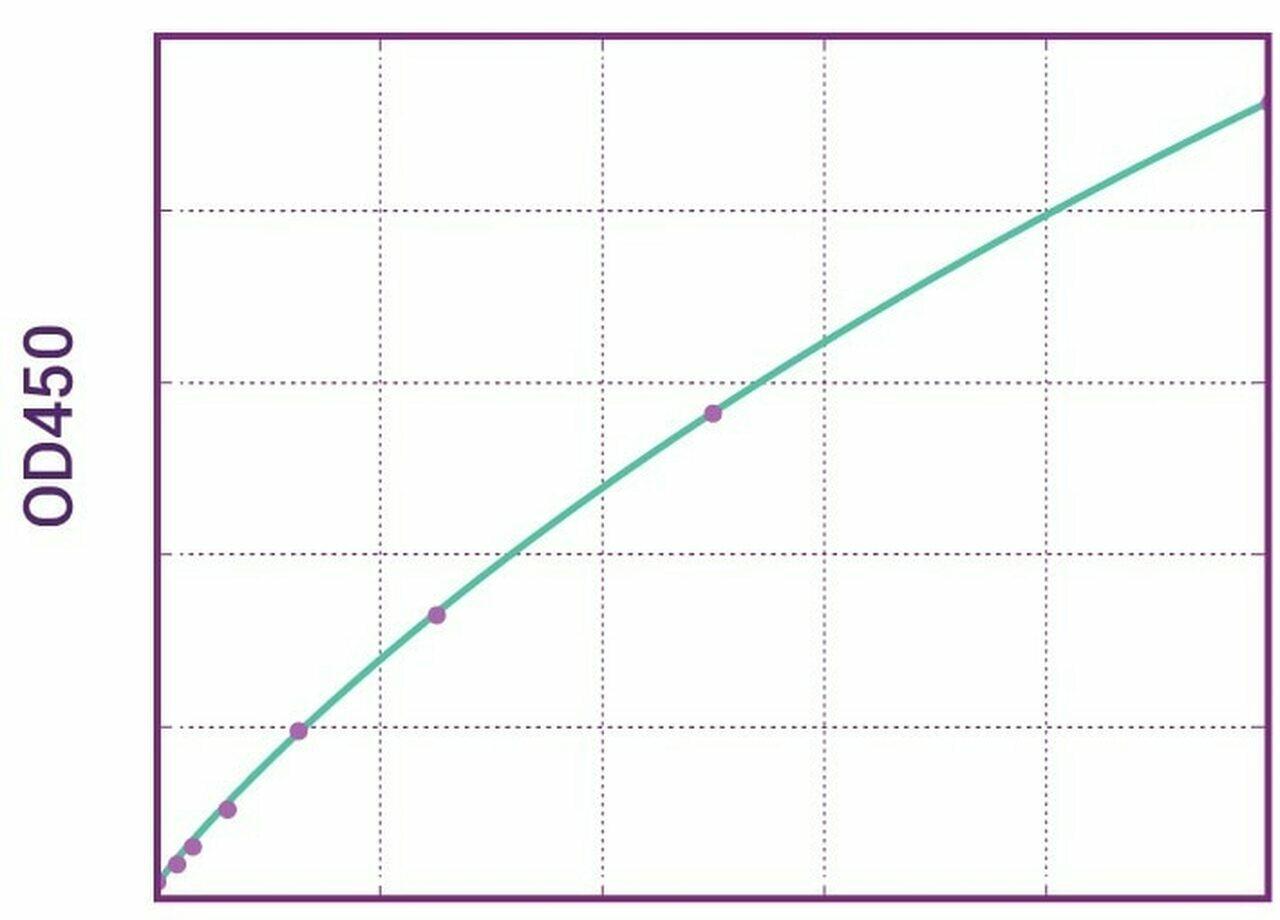
| Anti-Avelumab (Bavencio®) ADA | |
|---|---|
| Product Code | HUMB00043 |
| ELISA Type | Antibody screening - Qualitative |
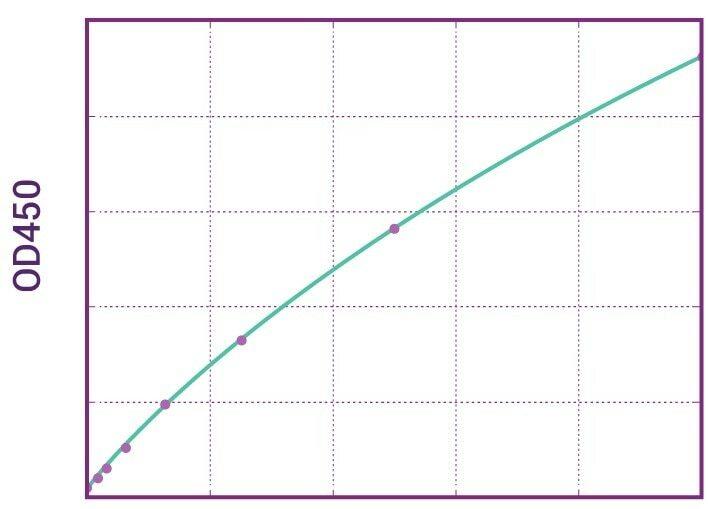
| Anti-Nivolumab (Opdivo®) ADA | |
|---|---|
| Product Code | HUMB00045 |
| ELISA Type | Antibody screening - Qualitative |
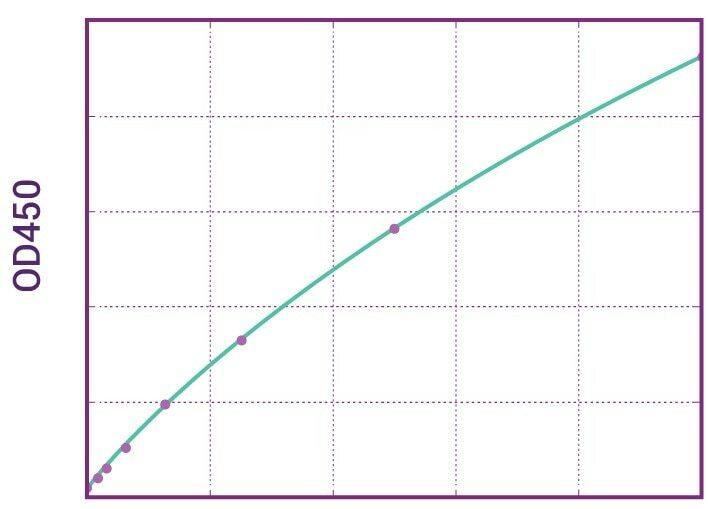
| Anti-Pembrolizumab (Keytruda®) ADA | |
|---|---|
| Product Code | HUMB00047 |
| ELISA Type | Antibody screening - Qualitative |
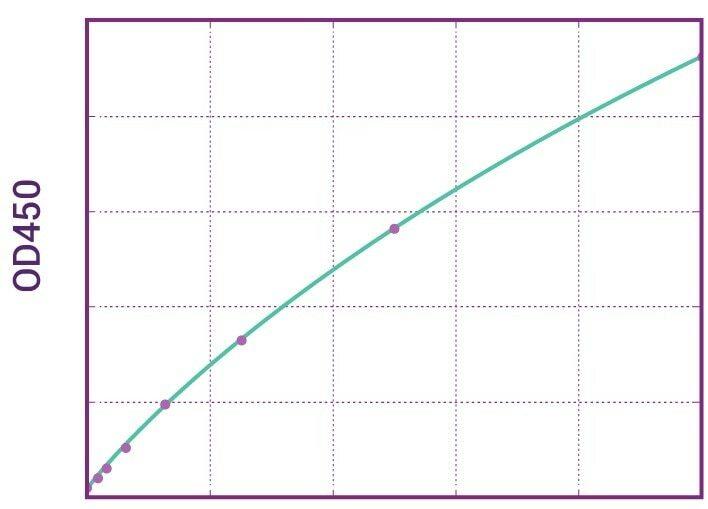
| Anti-Ipilimumab (Yervoy®) ADA | |
|---|---|
| Product Code | HUMB00049 |
| ELISA Type | Antibody screening - Qualitative |
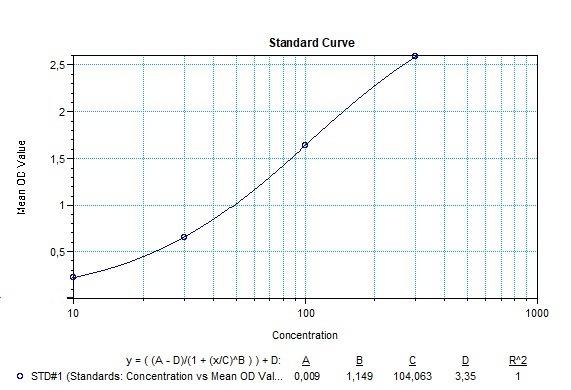
| Atezolizumab (Tecentriq®) ADA | |
|---|---|
| Product Code | HUMB00061 |
| ELISA Type | Antibody screening - Qualitative |
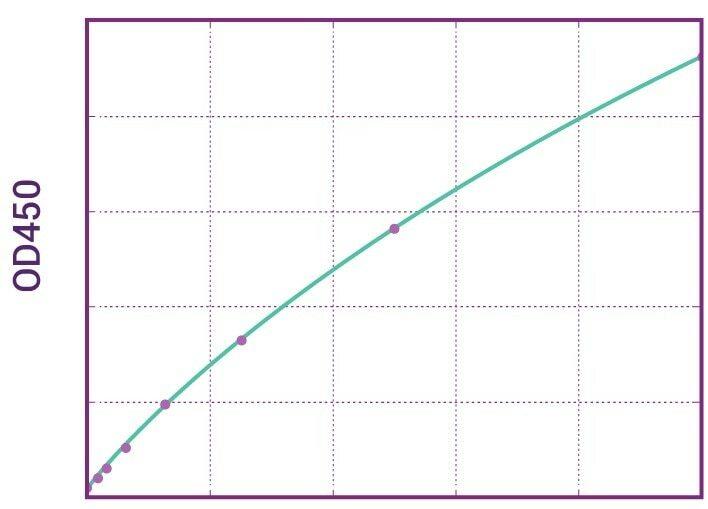
| Anti-Vedolizumab (Entyvio®) ADA Qualitative ELISA Kit | |
|---|---|
| Product Code | HUMB00019 |
| ELISA Type | Antibody screening - Qualitative |
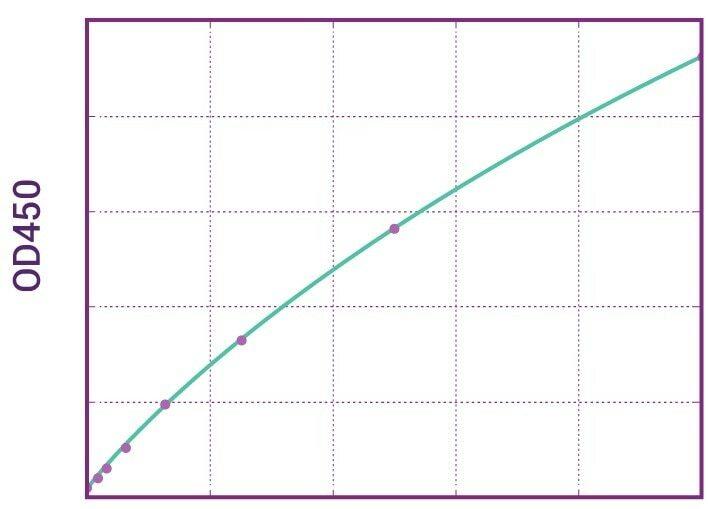
| Anti-Ustekinumab (Stelara®) ADA | |
|---|---|
| Product Code | HUMB00021 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Tocilizumab (Actemra®) | |
|---|---|
| Product Code | HUMB00051 |
| ELISA Type | Antibody screening - Qualitative |
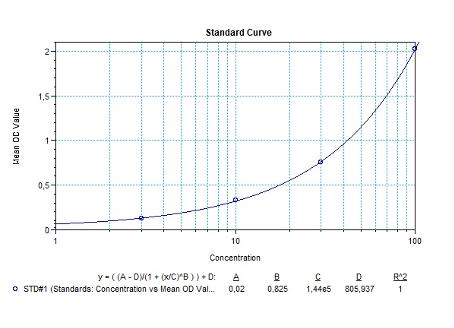
| Anti-Natalizumab (Tysabri®) ADA | |
|---|---|
| Product Code | HUMB00053 |
| ELISA Type | Antibody screening - Qualitative |
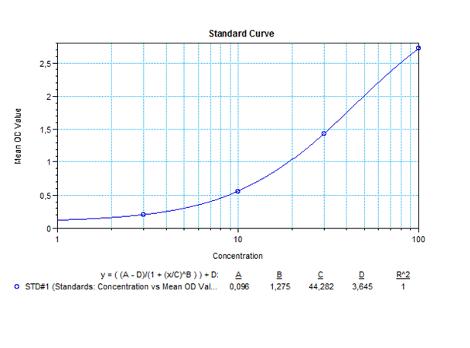
| Anti-Canakinumab (Ilaris®) ADA | |
|---|---|
| Product Code | HUMB00057 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Abatacept (Orencia®) ADA | |
|---|---|
| Product Code | HUMB00069 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Secukinumab (Cosentyx® , Verxant®) ADA | |
|---|---|
| Product Code | HUMB00067 |
| ELISA Type | Antibody screening - Qualitative |
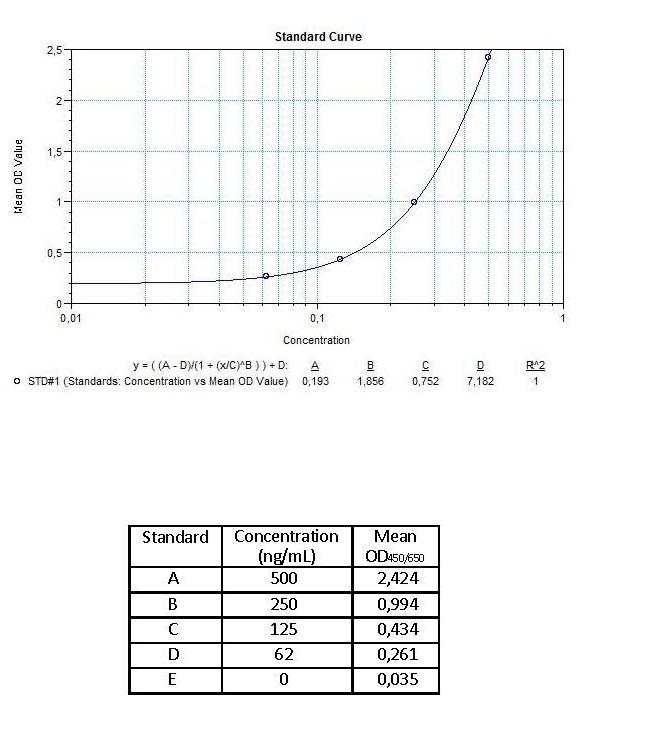
| Anti-Infliximab (Remicade®) ADA | |
|---|---|
| Product Code | HUMB00002 |
| ELISA Type | Antibody screening - Qualitative |
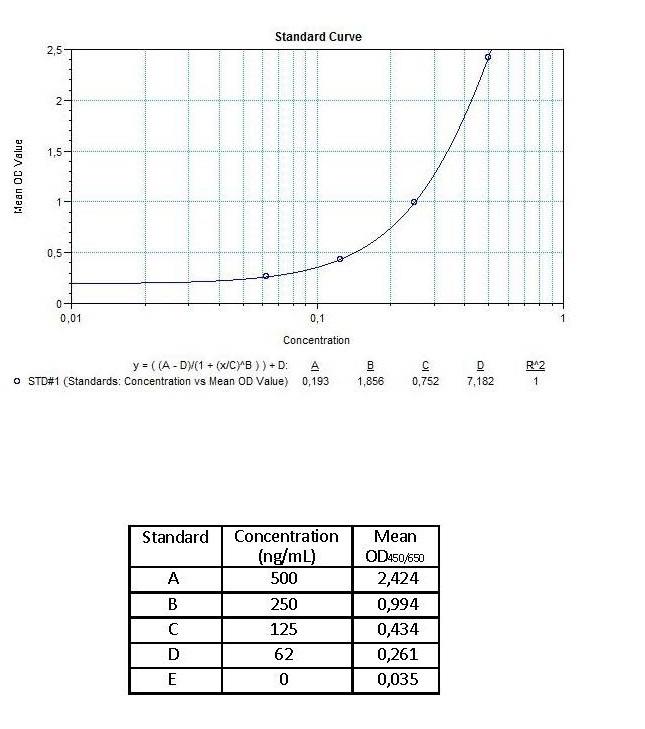
| Anti-Infliximab (Remicade®) ADA | |
|---|---|
| Product Code | HUMB00003 |
| ELISA Type | Antibody screening - Quantitative |
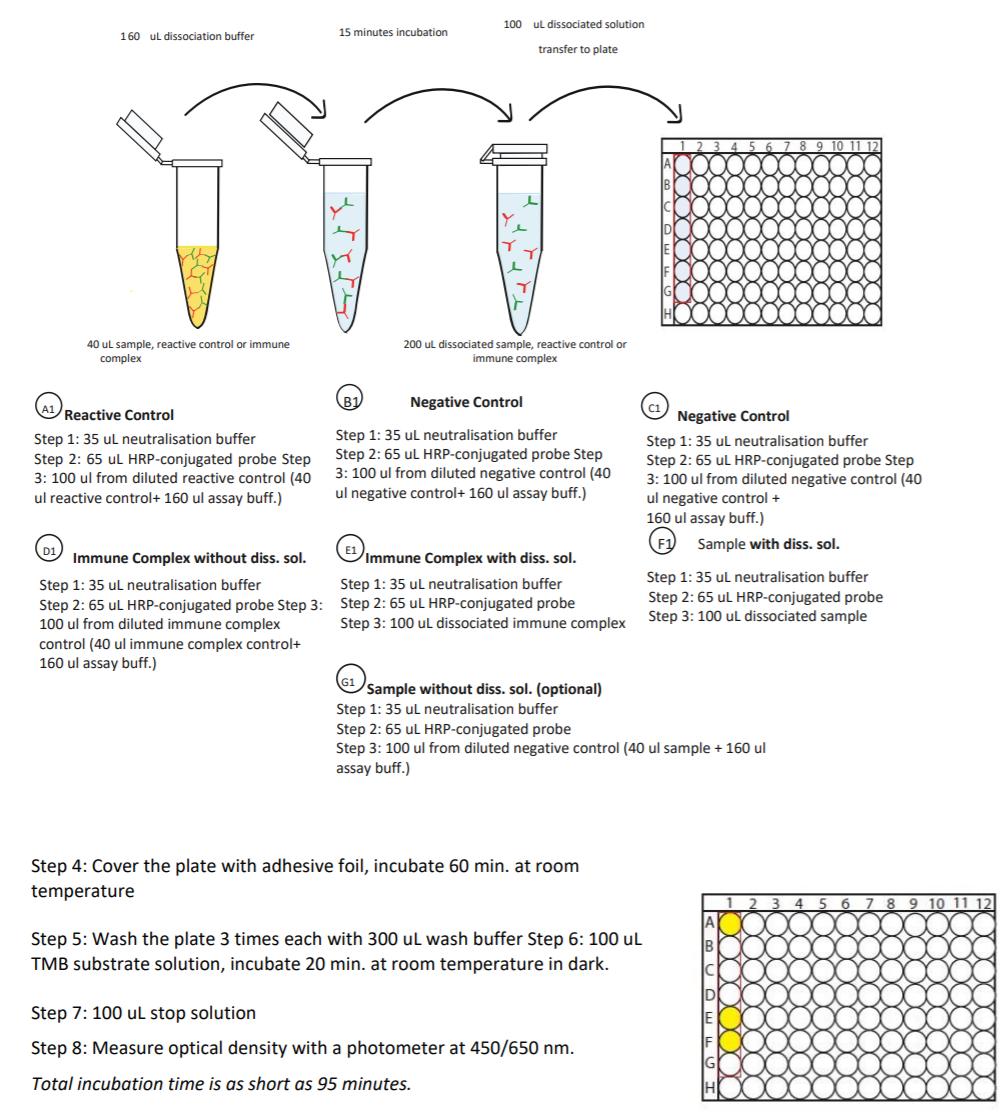
| Anti-Infliximab (Remicade®) Free Drug/ADA Dual ELISA | |
|---|---|
| Product Code | HUMB00004 |
| ELISA Type | Antibody screening - Free/Total semiquantitative |
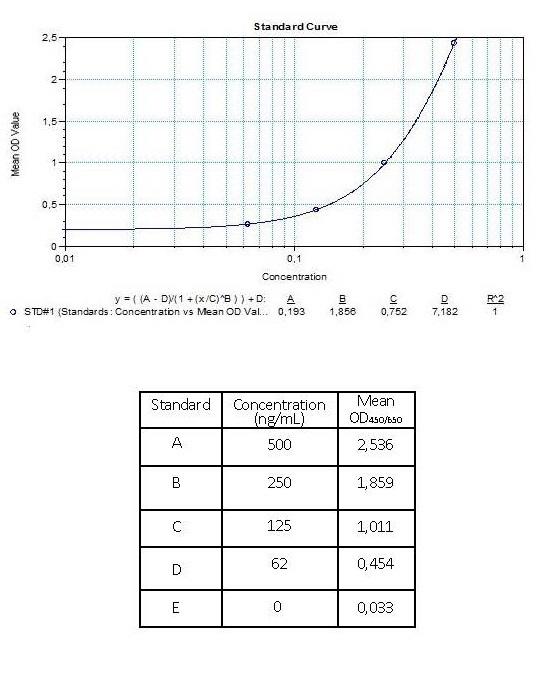
| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00006 |
| ELISA Type | Antibody screening - Qualitative |
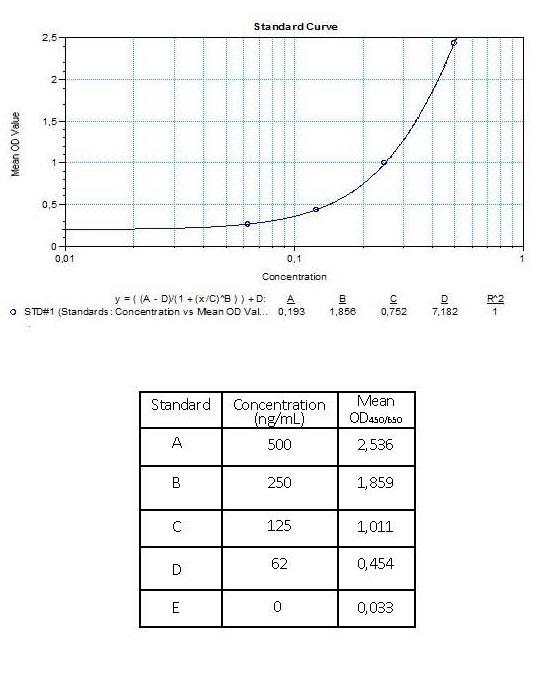
| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00007 |
| ELISA Type | Antibody screening - Quantitative |

| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00008 |
| ELISA Type | Antibody screening - Total semiquantitative |
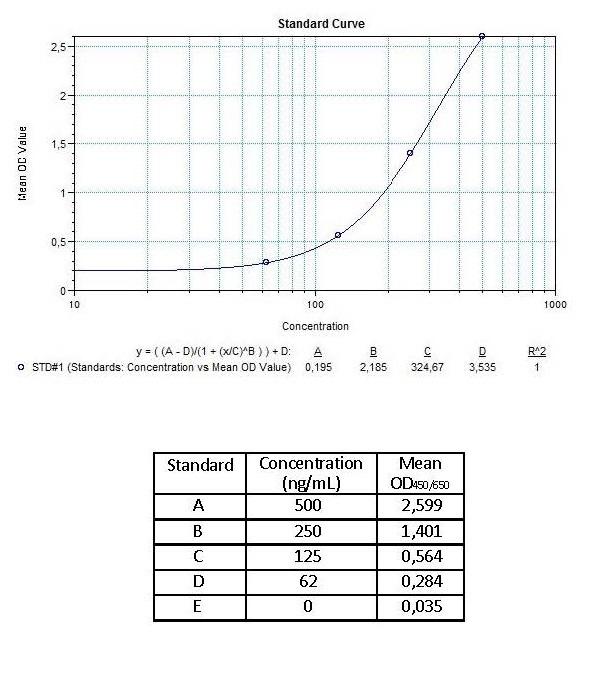
| Anti-Adalimumab (Humira®) ADA | |
|---|---|
| Product Code | HUMB00010 |
| ELISA Type | Antibody screening - Qualitative |
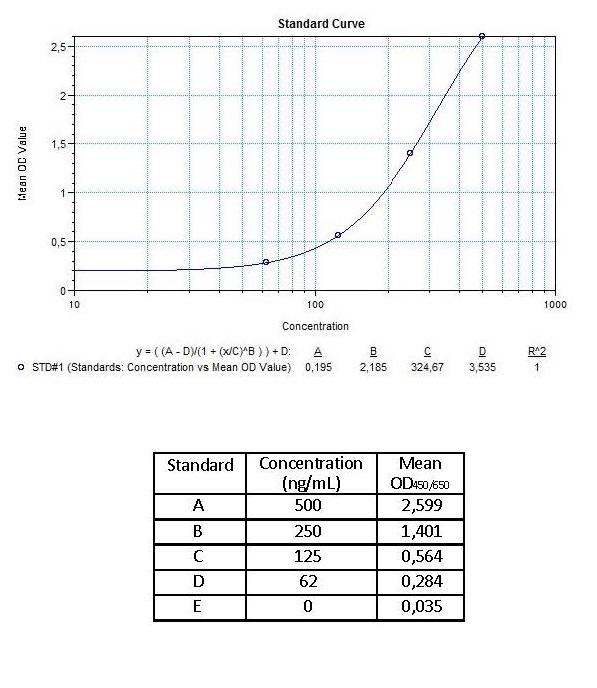
| Anti-Adalimumab (Humira®) ADA | |
|---|---|
| Product Code | HUMB00011 |
| ELISA Type | Antibody screening - Quantitative |
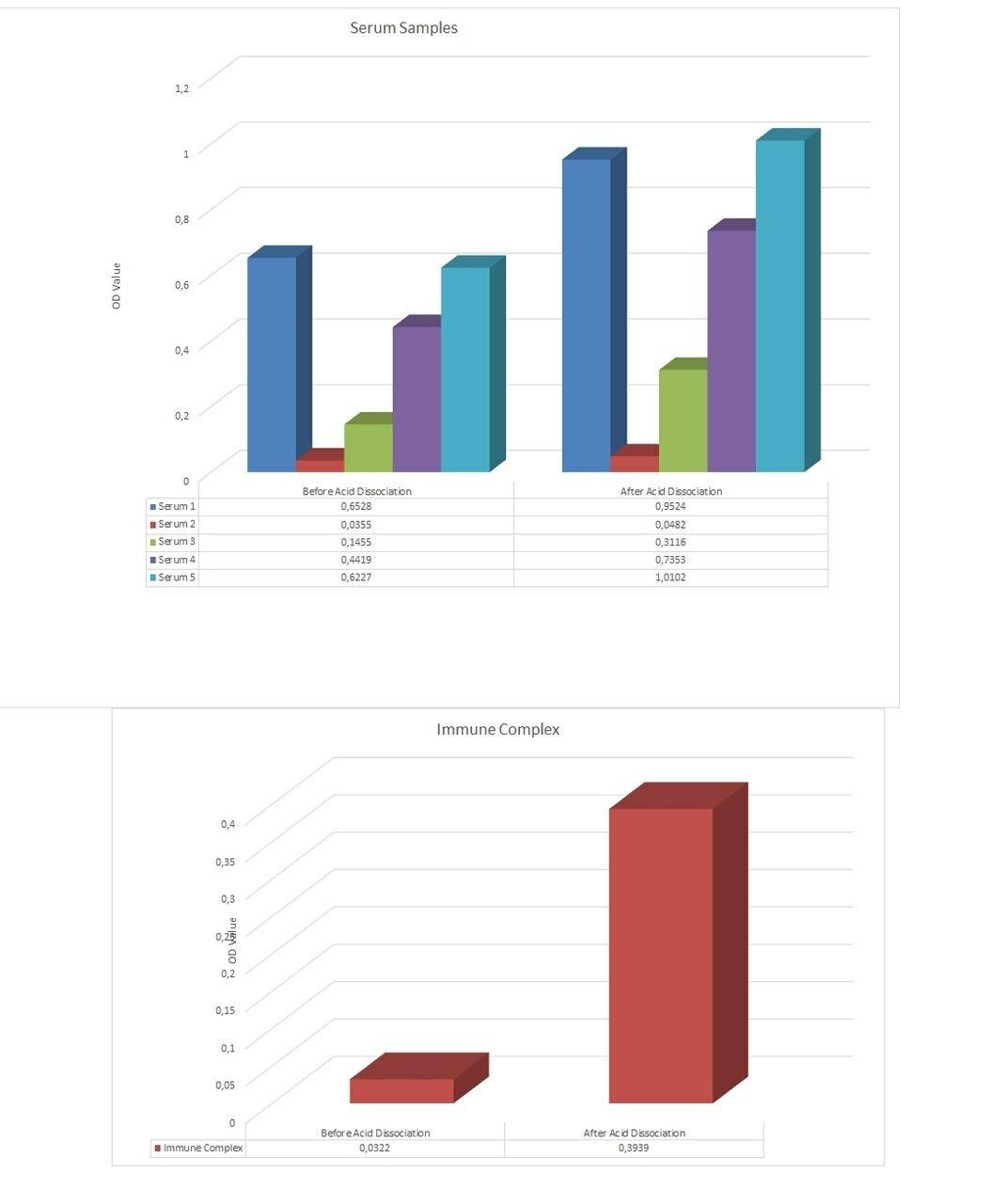
| Anti-Adalimumab (Humira®) Free Drug/ADA Dual ELISA | |
|---|---|
| Product Code | HUMB00012 |
| ELISA Type | Antibody screening - Free/Total semiquantitative |
| Anti-Etanercept (Enbrel®) ADA | |
|---|---|
| Product Code | HUMB00014 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Golimumab (Simponi®) ADA | |
|---|---|
| Product Code | HUMB00016 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |
| Anti-Omalizumab (Xolair®) ADA | |
|---|---|
| Product Code | HUMB00040 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Denosumab (Prolia®) ADA | |
|---|---|
| Product Code | HUMB00037 |
| ELISA Type | Antibody screening - Qualitative |
Additional Resources