Apamistamab: Advancing Leukemia Treatment through Targeted Radioimmunotherapy
Quick Facts About Apamistamab
What is Apamistamab?
Apamistamab is a monoclonal antibody that targets the CD45 antigen on hematopoietic cells.
How does Apamistamab work?
When labeled with the radioactive isotope Iodine-131, Apamistamab delivers targeted radiation to CD45-expressing cells, aiding in the eradication of malignant cells.
What are the clinical applications of Apamistamab?
Apamistamab is primarily used in conditioning regimens before hematopoietic cell transplantation for patients with relapsed or refractory acute myeloid leukemia (AML).
1.) Understanding Apamistamab
Apamistamab, also known as Iomab-B when conjugated with Iodine-131, is an innovative therapeutic agent designed to improve outcomes for patients with hematologic malignancies, particularly acute myeloid leukemia (AML). By targeting the CD45 antigen—a protein expressed on all hematopoietic cells but limited in non-hematopoietic tissues—Apamistamab delivers cytotoxic radiation directly to malignant cells while sparing healthy tissues.
This targeted approach addresses the limitations of traditional conditioning regimens, which often involve non-specific chemotherapy and radiation, leading to significant toxicity. Standard conditioning treatments rely on high-dose chemotherapy and total body irradiation (TBI) to eradicate malignant cells and suppress the immune system before hematopoietic stem cell transplantation (HSCT). However, these methods frequently cause severe side effects, including damage to vital organs such as the liver, lungs, and gastrointestinal tract, as well as increased risks of infection and graft-versus-host disease (GVHD).
Apamistamab’s specificity for CD45 allows for a more focused treatment, reducing collateral damage and potentially improving patient outcomes. Unlike TBI, which exposes the entire body to radiation, Apamistamab selectively delivers radiation to hematopoietic tissues, effectively eliminating cancerous cells while minimizing exposure to non-target organs. This selectivity is crucial for patients with relapsed or refractory AML who may not tolerate the toxicity of conventional conditioning regimens.
Furthermore, Apamistamab has been shown to enhance the likelihood of successful engraftment following HSCT by efficiently clearing residual malignant cells. By reducing toxicity and improving the efficacy of transplantation, Apamistamab represents a significant advancement in leukemia treatment, offering new hope for patients with limited therapeutic options.
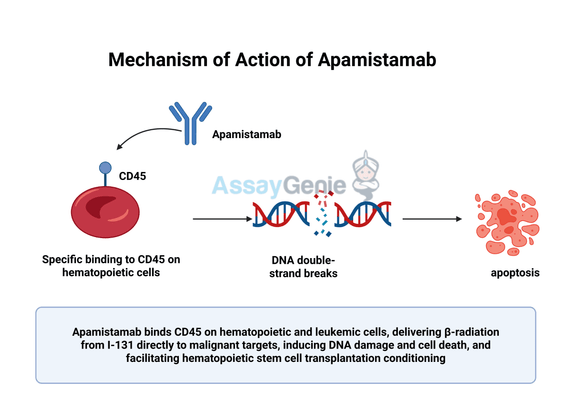
2.) Mechanism of Action of Apamistamab
Apamistamab functions as a radioimmunoconjugate, combining the specificity of a monoclonal antibody with the cytotoxic power of targeted radiation. As a monoclonal antibody, Apamistamab specifically binds to CD45, a transmembrane glycoprotein expressed on nearly all hematopoietic cells, including leukemic blasts in acute myeloid leukemia (AML). However, CD45 is minimally expressed on non-hematopoietic tissues, making it an ideal target for selective treatment.
Upon administration, Apamistamab circulates in the bloodstream and binds to CD45-expressing cells in the bone marrow, spleen, and other hematopoietic tissues. The attached Iodine-131 isotope, a beta-emitting radionuclide, delivers continuous, localized radiation to the targeted cells. Beta radiation penetrates only a short distance, ensuring that the cytotoxic effects are concentrated on malignant cells while sparing adjacent healthy tissues. The emitted radiation induces DNA double-strand breaks and other forms of DNA damage, leading to apoptosis and cell death in cancerous cells.
This targeted mechanism allows for the efficient delivery of high-dose radiation directly to leukemic cells while minimizing systemic toxicity. The precision of Apamistamab is particularly beneficial in conditioning patients for hematopoietic stem cell transplantation (HSCT), as it effectively eliminates residual malignant cells and reduces the burden of disease before transplantation. Additionally, by selectively depleting host hematopoietic cells, Apamistamab facilitates optimal engraftment of donor stem cells, increasing the likelihood of successful transplantation and reducing the risk of disease relapse. This targeted approach represents a significant advancement over traditional conditioning regimens that rely on systemic chemotherapy and total body irradiation, which often cause severe side effects and damage to healthy organs.
3.) Clinical Applications of Apamistamab
Apamistamab has emerged as a promising therapeutic agent in conditioning regimens for patients undergoing hematopoietic cell transplantation (HCT), particularly those with relapsed or refractory acute myeloid leukemia (AML). Traditional conditioning regimens, which involve high-dose chemotherapy and total body irradiation, often come with significant toxicity and are unsuitable for patients with active disease or comorbidities. Apamistamab, when conjugated with Iodine-131 (Iomab-B), offers a targeted alternative by selectively delivering radiation to CD45-expressing hematopoietic cells while sparing non-hematopoietic tissues.
The efficacy of Apamistamab has been demonstrated in clinical trials, most notably the Phase 3 SIERRA trial, which evaluated its use in older patients with active, relapsed, or refractory AML who were considered ineligible for standard transplantation conditioning regimens. The trial showed that patients treated with Apamistamab successfully achieved myeloablation and donor engraftment, leading to improved overall survival compared to conventional approaches. By selectively eradicating malignant cells and minimizing toxicity to healthy tissues, Apamistamab has expanded transplant eligibility to high-risk patients who otherwise had limited treatment options.
Beyond AML, Apamistamab's targeted approach has potential applications in other hematologic malignancies, such as myelodysplastic syndromes (MDS) and certain lymphomas, where CD45 expression is prevalent. Its ability to enhance the safety and efficacy of transplantation makes it a valuable tool in the evolving landscape of precision medicine. As further research continues, Apamistamab may redefine conditioning regimens, offering improved outcomes and quality of life for patients undergoing stem cell transplantation.

4.) Advancing Research on Apamistamab
The development of biosimilar products for Apamistamab presents significant opportunities to advance research and improve accessibility.
What is a Biosimilar?
A biosimilar is a biologic medical product highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, purity, and potency. Biosimilars offer cost-effective alternatives, facilitating broader access to advanced therapies and promoting further research and innovation.
| Apamistamab (Anti-CD45) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD45 |
| Reactivity: | Human |
Comparison: Apamistamab and Its Biosimilar
Biosimilars of Apamistamab are designed to mirror the original product's structure, function, and clinical efficacy. They target the same CD45 antigen and are conjugated with Iodine-131 to deliver targeted radiation to hematopoietic cells. Rigorous analytical and clinical studies ensure that these biosimilars match the reference product in quality and performance.
Benefits for Research Applications
The availability of Apamistamab biosimilars enhances research by providing more accessible tools for studying targeted radioimmunotherapy in hematologic malignancies. Researchers can utilize these biosimilars to explore new therapeutic combinations, dosing strategies, and potential applications in other CD45-expressing malignancies.
Research Use Only Disclaimer:
It is important to note that Apamistamab biosimilars are intended for research use only and are not approved for clinical applications. Researchers must adhere to appropriate guidelines and regulations when utilizing these products in preclinical studies.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026