Cleaved Caspase-3 and Apoptosis
Delve into the role of Cleaved Caspase-3 in programmed cell death and its implications in disease pathologies, unraveling its regulatory mechanisms and potential as a therapeutic target.
Key Takeaways:
- Cleaved Caspase-3 is a central enzyme in apoptosis, orchestrating cell dismantling.
- It's activated by initiator caspases and is key in executing the apoptotic process.
- Cleaved Caspase-3 has roles in diseases like cancer and neurodegenerative disorders.
- Its regulation involves various factors, including IAPs and the Bcl-2 family.
- Targeting Cleaved Caspase-3 offers potential therapeutic strategies for various diseases.
Table of Contents
Jump to a section:
Cleaved Caspase-3
Programmed cell death, known as apoptosis, is a tightly regulated process crucial for various physiological functions, including tissue homeostasis and development. Caspases, a family of enzymes, play a central role in orchestrating apoptosis. Among them, Caspase-3 stands out as a key executioner, responsible for initiating and executing the ordered breakdown of cellular components. Upon activation, Caspase-3 undergoes proteolytic cleavage, giving rise to Cleaved Caspase-3, the active form critical for mediating apoptosis.
What are Caspases?
Caspases are a family of cysteine proteases that play a central role in programmed cell death, known as apoptosis. They are essential regulators and mediators of the apoptotic process, orchestrating the orderly dismantling of cells.
There are two main groups of caspases: initiator caspases (e.g., Caspase-8 and Caspase-9) and executioner caspases (e.g., Caspase-3). Initiator caspases sense and transmit apoptotic signals, while executioner caspases carry out the actual cell breakdown.
Among the caspases, Caspase-3 stands out as a specific effector caspase. Upon initiation of apoptosis, Caspase-3 is cleaved and activated, propagating the apoptotic signal by targeting downstream substrates like poly ADP ribose polymerase (PARP) and other cellular proteins.
Researchers often employ Caspase-3 antibodies to detect both the uncleaved and cleaved forms of the enzyme, making them strong indicators of cell death induction in cell biology studies. These antibodies are widely used to assess the cytotoxic potency and effectiveness of potential therapeutic agents.
Caspase-3 Structure
Caspase-3 is a protein enzyme composed of 277 amino acids, organized into two subunits: a large subunit (p20) and a small subunit (p10). These subunits are connected by a flexible linker region, forming the inactive proenzyme, also known as procaspase-3. The catalytic activity of Caspase-3 is contained within a pocket at the junction of its p20 and p10 subunits. This pocket houses an active site cysteine residue that is critical for the proteolytic cleavage of specific substrates. Caspase-3 targets proteins at a conserved aspartic acid residue, recognizing and cleaving specific substrate sequences. In its inactive state, Caspase-3 cannot execute proteolytic cleavage. Instead, it requires activation through proteolytic processing at specific cleavage sites.
Caspase-3 Activation and Formation of Cleaved Caspase-3
The activation of Caspase-3 is a tightly regulated process and is triggered in response to various apoptotic signals. Initiator caspases, such as Caspase-8 or Caspase-9, initiate this activation process in response to apoptotic signals. These initiator caspases are activated by upstream signals, including extrinsic death ligands (e.g., TNF-alpha) or intrinsic mitochondrial signals (e.g., cytochrome c release). Once activated, initiator caspases cleave and activate Caspase-3 at specific sites within the proenzyme.
Upon activation, Caspase-3 undergoes a conformational change, leading to the dissociation of its proenzyme form into two subunits: a large subunit (p20) and a small subunit (p10). This proteolytically processed form of Caspase-3 is referred to as Cleaved Caspase-3 or active Caspase-3. Cleaved Caspase-3 exhibits enzymatic activity, enabling it to carry out its function as an executioner caspase in the apoptotic pathway.
Cleaved Caspase-3 in Apoptosis
Cleaved Caspase-3 plays a critical role in the execution phase of apoptosis, orchestrating the ordered dismantling of cells. Its function and activity are essential for carrying out the irreversible process of programmed cell death.
-
Initiating the Execution Phase:
During apoptosis, the activation of Cleaved Caspase-3 marks the initiation of the execution phase. Upon receiving apoptotic signals, initiator caspases, such as Caspase-8 or Caspase-9, cleave and activate Caspase-3, transforming it into the active Cleaved Caspase-3 form. This activation serves as a point of no return, setting in motion a cascade of events that lead to the efficient destruction of the cell. -
Proteolytic Activity:
Cleaved Caspase-3 is an executioner caspase, meaning it targets and cleaves specific cellular substrates with aspartate residues in their recognition sequences. By proteolytically cleaving these substrates, Cleaved Caspase-3 initiates a series of cellular changes that are characteristic of apoptosis. These changes include chromatin condensation, DNA fragmentation, and the disassembly of structural components within the cell. -
Ensuring Cellular Dismantling:
Through its proteolytic activity, Cleaved Caspase-3 facilitates the dismantling of the cell in an ordered and controlled manner. The targeted cleavage of essential cellular proteins leads to the breakdown of the cell's structural integrity, effectively eliminating its functional components. This dismantling process prevents the release of harmful cellular contents and ensures a clean and regulated elimination of the dying cell. -
Inducing Phagocytosis:
Cleaved Caspase-3 also plays a crucial role in promoting the efficient removal of apoptotic cells through a process called phagocytosis. As Cleaved Caspase-3 initiates the dismantling of the dying cell, it leads to the exposure of specific cell surface signals. These signals serve as recognition markers for neighboring cells or specialized phagocytes, guiding them to engulf and eliminate the apoptotic bodies in a controlled and organized manner. The prompt clearance of apoptotic cells helps to maintain tissue integrity and prevents the release of potentially harmful cellular contents, contributing to tissue homeostasis and proper functioning.
Cleaved Caspase-3 in Apoptosis
Cleaved Caspase-3 Related Kits
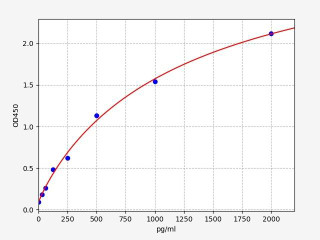
| Human Cleaved CASP8 (Cleaved Caspase-8) ELISA Kit (HUFI04741) | |
|---|---|
| ELISA TYPE | Sandwich |
| SENSITIVITY | 18.75pg/ml |
| RANGE | 31.25-2000pg/ml |
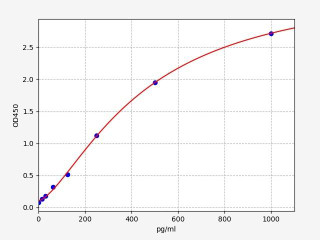
| Human Cleaved CASP9 (Cleaved Caspase-9) ELISA Kit (HUFI04740) | |
|---|---|
| ELISA TYPE | Sandwich |
| SENSITIVITY | 9.375pg/ml |
| RANGE | 15.625-1000pg/ml |
Regulation of Caspase-3 Activation
The activation of Caspase-3, a critical event in apoptosis, is subject to strict regulatory control to ensure the precise and timely execution of programmed cell death. Various factors and signaling pathways converge to initiate the activation of initiator caspases, which subsequently activate Caspase-3 through proteolytic cleavage.
-
Initiator Caspases and Signal Integration:
The activation of Caspase-3 is initiated by upstream initiator caspases, including Caspase-8 and Caspase-9, which are activated in response to specific apoptotic signals. These initiator caspases act as pivotal integrators of extrinsic and intrinsic apoptotic pathways, integrating environmental cues and cellular stress signals to coordinate Caspase-3 activation. -
Ubiquitin-Mediated Regulation:
Ubiquitin-dependent regulatory mechanisms also modulate Caspase-3 activation. Ubiquitin ligases, such as cIAPs (cellular inhibitor of apoptosis proteins) and the E3 ubiquitin ligase CHIP (carboxyl terminus of Hsc70-interacting protein), can promote or inhibit Caspase-3 activation through ubiquitination of specific substrates. The balance between ubiquitination and deubiquitination events delicately regulates Caspase-3 activation and the extent of apoptosis. -
Inhibitor of Apoptosis Proteins (IAPs):
The Inhibitors of Apoptosis Proteins (IAPs), including XIAP and survivin, are negative regulators of apoptosis that bind and inhibit caspases, including Caspase-3. Smac/DIABLO (Second mitochondria-derived activator of caspases/Direct IAP-Binding Protein with Low PI), a pro-apoptotic protein released during apoptosis, antagonizes IAPs, promoting Caspase-3 activation and apoptosis. -
Pro-apoptotic Bcl-2 Family Members:
The balance between pro-apoptotic and anti-apoptotic members of the Bcl-2 family also influences Caspase-3 activation. Pro-apoptotic members, such as Bax and Bak, facilitate the release of cytochrome c from mitochondria, a crucial event in apoptosome formation and Caspase-3 activation.
Role of Cleaved Caspase-3 in Disease
The pivotal role of Cleaved Caspase-3 in apoptosis extends beyond its significance as a marker for cell death induction. Emerging evidence implicates Cleaved Caspase-3 in various diseases and medical conditions, highlighting its potential as a therapeutic target. Understanding the involvement of Cleaved Caspase-3 in disease pathogenesis offers valuable insights into disease mechanisms and therapeutic strategies.
Cleaved Caspase-3 and Cancer
Dysregulation of apoptosis is a hallmark of cancer, enabling tumor cells to evade cell death and promote uncontrolled growth. In cancer cells, increased levels of Cleaved Caspase-3 have been observed, indicating ongoing apoptotic events that might be hijacked by pro-survival pathways. Targeting Cleaved Caspase-3 and other components of the apoptotic pathway represents a promising approach for cancer therapy. Induction of apoptosis in cancer cells through targeted therapy can potentially inhibit tumor growth and sensitize cancer cells to conventional treatments, such as chemotherapy and radiation.
Cleaved Caspase-3 and Neurodegenerative Diseases
Neurodegenerative diseases, including Alzheimer's, Parkinson's, and Huntington's diseases, involve progressive loss of neurons and synaptic dysfunction. Emerging research suggests that Cleaved Caspase-3 is involved in the neurodegenerative process. Elevated levels of Cleaved Caspase-3 have been detected in affected brain regions, indicating neuronal apoptosis. Identifying the precise role of Cleaved Caspase-3 in neurodegeneration may lead to the development of novel therapeutic interventions aimed at preserving neuronal function and delaying disease progression.
Cleaved Caspase-3 and Cardiovascular Diseases
Caspase-3 plays a significant role in cardiovascular diseases, particularly in conditions involving cardiomyocyte apoptosis and adverse cardiac remodeling. During events like myocardial infarction and ischemia-reperfusion injury, increased apoptotic events in cardiomyocytes lead to the loss of functional heart muscle cells. Caspase-3 activation during ischemia and reperfusion exacerbates cellular damage, impairing cardiac function and potentially leading to heart failure. Targeting Caspase-3 activity shows promise as a therapeutic approach to limit cell death and improve outcomes in cardiovascular pathologies, offering new avenues for precise and effective treatments in heart-related disorders.
Written by Lauryn McLoughlin
Lauryn McLoughlin completed her undergraduate degree in Neuroscience before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026