Gemtuzumab: Advancing CD33-Targeted Therapies in AML Research
Key Facts About Gemtuzumab
What is Gemtuzumab?
Gemtuzumab is an antibody-drug conjugate (ADC) targeting CD33, a protein expressed on the surface of myeloid cells. It is widely recognized for its role in treating acute myeloid leukemia (AML).
How does Gemtuzumab work?
Gemtuzumab binds to CD33-positive cells and delivers a cytotoxic agent, ozogamicin, to induce cell death specifically in AML cells, sparing healthy tissue.
What is the approval history of Gemtuzumab Ozogamicin?
Initially approved by the FDA in 2000, Gemtuzumab ozogamicin (Mylotarg) faced a withdrawal in 2010. However, it was reapproved in 2017 after new data affirmed its efficacy and safety.
1.) Understanding Gemtuzumab
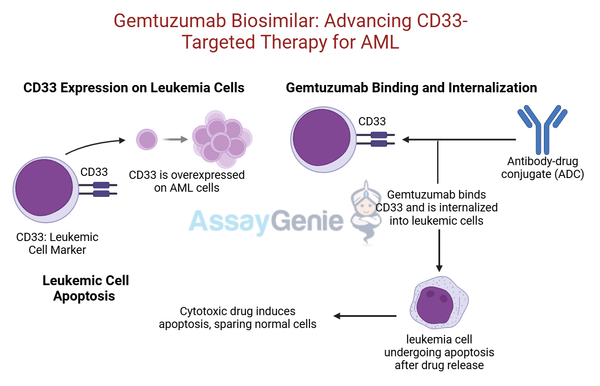
Gemtuzumab ozogamicin is a groundbreaking antibody-drug conjugate (ADC) that marked a significant advancement in targeted cancer therapy. As one of the first ADCs developed specifically for acute myeloid leukemia (AML), Gemtuzumab combines the precision of monoclonal antibody targeting with the potent cytotoxicity of chemotherapy. Its target, CD33, is a cell surface antigen expressed on leukemic blasts in the majority of AML cases, making it an ideal marker for therapeutic intervention.
Gemtuzumab's mechanism involves a monoclonal antibody that binds selectively to CD33 on AML cells. Once bound, the ADC is internalized, and its cytotoxic payload, a calicheamicin derivative, is released within the cancer cells. This payload induces double-stranded DNA breaks, ultimately leading to cell death. By delivering the cytotoxic agent directly to CD33-positive cells, Gemtuzumab minimizes off-target effects and reduces damage to healthy tissues, enhancing its safety profile compared to traditional chemotherapy.
This precision approach has positioned Gemtuzumab as a valuable treatment option for both newly diagnosed and relapsed/refractory AML cases. Its ability to selectively target leukemic cells while sparing normal hematopoietic cells has also paved the way for its use in combination therapies, further improving outcomes in difficult-to-treat AML patients. Gemtuzumab’s development represents a milestone in the evolution of ADCs and targeted oncology.
Prefer to Listen? Check out the Gemtuzumab Podcast Episode
2.) Mechanism of Action of Gemtuzumab
Gemtuzumab ozogamicin functions as a highly targeted therapy for acute myeloid leukemia (AML), leveraging its antibody-drug conjugate (ADC) design to specifically attack leukemic cells while minimizing harm to normal tissues. The process begins with the monoclonal antibody component of Gemtuzumab binding to CD33, a cell surface antigen highly expressed on the majority of AML cells. This targeted binding ensures precise delivery of the therapeutic agent to malignant cells.
Once Gemtuzumab binds to CD33, the antibody-drug complex is internalized into the cancer cell through receptor-mediated endocytosis. Inside the cell, the cytotoxic payload, ozogamicin—a derivative of the potent chemotherapy agent calicheamicin—is released in the acidic environment of the lysosome. Ozogamicin interacts with the cell’s DNA, causing double-strand breaks that disrupt replication and transcription. This DNA damage ultimately triggers apoptosis, leading to the destruction of the leukemic cell.
This targeted mechanism not only enhances the therapeutic efficacy of Gemtuzumab but also significantly reduces off-target effects and systemic toxicity compared to conventional chemotherapies. By sparing healthy cells from unnecessary damage, Gemtuzumab achieves a more favorable safety profile, offering AML patients a better quality of life during treatment. Its ability to selectively target CD33-expressing cells makes it an integral component of modern AML therapy, especially in combination with other agents.
3.) Clinical Applications of Gemtuzumab
Gemtuzumab ozogamicin has established itself as a pivotal therapy in the management of acute myeloid leukemia (AML), particularly in patients with CD33-positive tumors, which account for the majority of AML cases. Its targeted approach and efficacy have positioned it as an important option for both newly diagnosed and relapsed/refractory AML patients.
One of the primary applications of Gemtuzumab is as part of combination regimens with standard chemotherapy agents such as cytarabine and daunorubicin, collectively known as the “3+7” regimen. Clinical trials have demonstrated that incorporating Gemtuzumab into these regimens significantly improves overall survival rates and remission outcomes compared to chemotherapy alone. These benefits are particularly notable in patients with favorable and intermediate cytogenetic risk profiles, where the addition of Gemtuzumab enhances the depth and duration of remission.
Gemtuzumab is also used as a standalone treatment in specific patient populations, such as older adults or those who cannot tolerate intensive chemotherapy. In these cases, Gemtuzumab provides a less toxic yet effective alternative. Moreover, ongoing research is exploring its potential in minimal residual disease (MRD) settings to prevent relapse by targeting residual leukemic cells.
With its demonstrated ability to improve clinical outcomes, Gemtuzumab continues to play a crucial role in AML therapy, offering hope for better prognosis and long-term survival.

4.) Advancing Research on Gemtuzumab with Biosimilars
Our Gemtuzumab Biosimilar serves as a powerful tool for advancing CD33-targeted therapy research. As a high-quality biosimilar, it provides researchers with a cost-effective and reliable alternative to the original molecule, supporting innovation in AML treatments.
What is a Biosimilar?
A biosimilar is a biologic that mirrors the reference product in safety, purity, and potency. Biosimilars foster research by offering affordable access to advanced biological tools, thus driving therapeutic innovation.
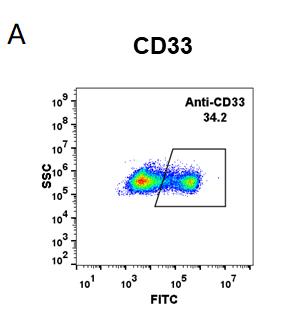
| Gemtuzumab (Anti-CD33) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD33 |
| Reactivity: | Human |
Benefits of the Gemtuzumab Biosimilar
Our biosimilar enables:
- Consistency: Ensures reproducibility and reliability in experimental outcomes.
- Cost-Effectiveness: Provides a budget-friendly option without compromising quality.
- Versatility: Suitable for diverse research applications, from preclinical studies to experimental drug development.
Research Use Only Disclaimer:
The Gemtuzumab Biosimilar is intended exclusively for research purposes and is not approved for clinical or therapeutic use. Researchers can explore its applications in experimental models to advance understanding of CD33-targeted therapies.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026