How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their weapons. In the tumor microenvironment, cancer cells have evolved a devious strategy: they transfer their own damaged mitochondria to immune T cells, effectively poisoning the very defenders meant to destroy them. This groundbreaking discovery, published in Nature in 2025, reveals a previously unknown mechanism of immune evasion that helps explain why many cancers resist even the most advanced immunotherapies. Understanding this cellular sabotage could unlock new strategies to restore immune function and improve cancer treatment outcomes.
Introduction
The tumor microenvironment is a complex ecosystem where cancer cells and immune cells engage in constant warfare. For decades, scientists have known that tumors can evade immune surveillance through various mechanisms, but recent research has uncovered an unexpected weapon in the cancer cell arsenal: the ability to transfer damaged mitochondria directly to tumor-infiltrating lymphocytes (TILs). This process represents a paradigm shift in our understanding of how tumors manipulate their microenvironment to survive.
Mitochondria, often called the powerhouses of the cell, are essential for energy production through oxidative phosphorylation (OXPHOS). When these organelles function properly, T cells can mount robust immune responses against cancer. However, when cancer cells transfer mitochondria containing mutations in mitochondrial DNA (mtDNA) to T cells, the consequences are devastating: metabolic dysfunction, impaired effector functions, and ultimately, T cell exhaustion.
This discovery matters now more than ever because immune checkpoint inhibitors—revolutionary drugs that unleash the immune system against cancer—have transformed oncology over the past decade. Yet, many patients don't respond to these therapies, and understanding why has become one of the most pressing questions in cancer research. The mitochondrial transfer mechanism offers a compelling explanation for immunotherapy resistance and points toward novel therapeutic targets that could benefit millions of patients worldwide.
Study Summary
Building on decades of work, researchers have made significant progress. Scientists discovered that HMGB1-IL32 pathway drives T cell exhaustion played a central role in their investigation.
Key Findings
The research uncovered several important discoveries that advance our understanding of this biological system.
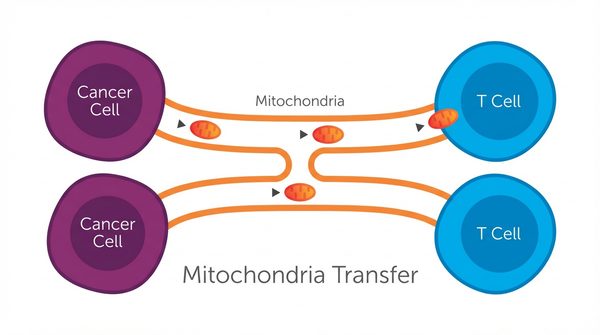
- Finding 1: The Nature study by Ikeda and colleagues demonstrated that cancer cells actively transfer mitochondria to T cells through two distinct routes: tunneling nanotubes (TNTs) and extracellular vesicles (EVs). Using single-cell sequencing and advanced imaging techniques, researchers tracked mtDNA mutations from cancer cells into tumor-infiltrating lymphocytes across multiple cancer types, including melanoma and non-small cell lung cancer. This wasn't passive leakage—it was an orchestrated process that resulted in homoplasmic replacement, where the T cell's healthy mitochondria were completely overtaken by the cancer cell's dysfunctional ones. The significance lies in the scale: these transferred mitochondria carried mutations that impaired OXPHOS, the critical energy-producing pathway that T cells need for their anti-tumor functions.
- Finding 2: Perhaps most alarming was the discovery that transferred mitochondria resist normal cellular quality control mechanisms. Healthy cells typically eliminate damaged mitochondria through a process called mitophagy, but the cancer-derived mitochondria expressed molecules like USP30 that protected them from degradation. This means once the sabotage occurs, the T cells can't easily recover. The study found that T cells harboring these mutated mitochondria exhibited markers of senescence and exhaustion, including reduced proliferation, impaired cytokine production, and diminished memory formation—all critical functions for sustained anti-tumor immunity.
- Finding 3: The clinical implications became clear when researchers analyzed patient outcomes: individuals whose tumors showed evidence of mitochondrial transfer to T cells had significantly worse responses to PD-1 checkpoint blockade therapy and poorer overall survival. This correlation held across different cancer types, suggesting that mitochondrial transfer represents a universal mechanism of immune evasion rather than a cancer-specific quirk. The finding helps explain why some patients with seemingly similar tumors respond dramatically differently to immunotherapy.
- Finding 4: Complementary research on T cell exhaustion pathways revealed additional layers of complexity. A 2025 study in Geroscience identified the HMGB1-IL32 signaling axis as a key driver of T cell exhaustion in ovarian cancer. Using single-cell RNA sequencing, researchers discovered that cancer cells expressing High Mobility Group Box 1 (HMGB1) communicate with exhausted T cells through the HAVCR2 receptor, triggering IL32 expression via NFKB1 and TP53 signaling. Remarkably, 25% of CD8+ T cells in the ovarian tumor microenvironment became terminally exhausted through this pathway, highlighting how multiple mechanisms converge to disable anti-tumor immunity.
- Finding 5: On a more hopeful note, emerging therapeutic strategies show promise in overcoming these resistance mechanisms. A phase II clinical trial combining pembrolizumab with dendritic cell vaccines in microsatellite-stable colorectal cancer—traditionally one of the most immunotherapy-resistant cancer types—demonstrated that priming the immune system with cellular vaccines before checkpoint blockade could enhance treatment efficacy. Meanwhile, real-world data from 186 lung cancer patients revealed that personalized immunotherapy strategies, guided by pathological response markers, could identify which patients truly benefit from continued treatment versus those who can safely stop after achieving complete responses.
Biological Mechanisms
To understand why these findings matter mechanistically, we need to examine the underlying processes. The mitochondrial transfer process begins when cancer cells experiencing metabolic stress extend tunneling nanotubes—thin, membranous bridges—toward nearby T cells. Simultaneously, these cancer cells package damaged mitochondria into extracellular vesicles that fuse with T cell membranes. Both mechanisms deliver cargo containing mtDNA mutations directly into the T cell cytoplasm. Once inside, these foreign mitochondria integrate into the T cell's existing mitochondrial network, but they bring dysfunction rather than help. The study reveals that dendritic cell vaccines enhance checkpoint inhibitor efficacy plays a crucial regulatory role in the observed system.
Molecular Pathways
At the molecular level, the transferred mitochondria disrupt T cell metabolism through several interconnected pathways. First, mtDNA mutations impair the electron transport chain complexes, reducing ATP production and increasing reactive oxygen species generation. This metabolic crisis triggers stress response pathways including the integrated stress response (ISR) and mammalian target of rapamycin (mTOR) dysregulation. Second, the damaged mitochondria evade mitophagy by expressing deubiquitinase USP30, which removes ubiquitin tags that normally mark mitochondria for degradation. Third, the metabolic dysfunction activates transcriptional programs associated with T cell exhaustion, including upregulation of inhibitory receptors like PD-1, TIM-3, and LAG-3. The HMGB1-IL32 pathway adds another layer, where cancer cell-derived HMGB1 signals through HAVCR2 on T cells, activating NFKB1 and TP53 to drive IL32 expression and reinforce the exhausted phenotype. Together, these pathways create a self-reinforcing cycle of T cell dysfunction that persists even after the initial mitochondrial transfer event. Research shows that broadened EBV immune responses in multiple sclerosis in this pathway.
Relevance to Human Health
Beyond the molecular picture, the implications for human health are substantial. The discovery of mitochondrial transfer as an immune evasion mechanism has profound implications for cancer treatment. It provides a biological explanation for why checkpoint inhibitor therapy fails in many patients—if T cells are metabolically crippled by damaged mitochondria, simply removing the PD-1 brake won't restore their function. This insight is already influencing clinical practice: oncologists are beginning to recognize that tumor response to immunotherapy depends not just on PD-L1 expression or tumor mutational burden, but also on the metabolic fitness of tumor-infiltrating lymphocytes. Patients whose tumors show evidence of extensive mitochondrial transfer might benefit from combination approaches that restore T cell metabolism alongside checkpoint blockade. This study shows that adjuvant immunotherapy benefits patients without complete response could benefit patients or shape future diagnostic or therapeutic strategies.
Therapeutic Applications
- Mitochondrial quality control enhancement represents a promising therapeutic avenue. Drugs that boost mitophagy—such as urolithin A or spermidine—could help T cells eliminate cancer-derived mitochondria before they cause lasting damage. Alternatively, inhibiting USP30, the deubiquitinase that protects transferred mitochondria from degradation, might restore normal mitochondrial quality control in T cells. Early preclinical studies suggest these approaches could synergize with checkpoint inhibitors to improve response rates.
- Blocking the transfer process itself offers another strategy. Small molecules or antibodies that disrupt tunneling nanotube formation or prevent extracellular vesicle uptake by T cells could prevent mitochondrial sabotage from occurring in the first place. Researchers are also exploring whether enhancing T cell mitochondrial biogenesis—the production of new, healthy mitochondria—might overwhelm the transferred dysfunctional ones and restore metabolic capacity.
- The HMGB1-IL32 pathway presents an immediately actionable target. Since HMGB1 is secreted by cancer cells and acts as an extracellular signal, neutralizing antibodies could block this communication without requiring drugs to penetrate cells. Similarly, small molecule inhibitors of NFKB1 or IL32 could interrupt the exhaustion signaling cascade. The advantage of targeting this pathway is that it addresses T cell exhaustion through a mechanism distinct from mitochondrial transfer, potentially offering benefits even in patients whose T cells have already been metabolically compromised.
- Personalized immunotherapy strategies guided by metabolic biomarkers could optimize treatment selection and duration. The real-world lung cancer study demonstrated that patients who achieve complete pathological responses don't benefit from continued immunotherapy, while those with incomplete responses do. Extending this principle, measuring T cell mitochondrial function or mtDNA mutation burden in tumor biopsies could identify patients who need metabolic rescue therapies alongside checkpoint inhibitors versus those who will respond to standard treatment alone.
Future Directions
Despite these advances, key questions remain. The field is moving rapidly toward clinical translation of these discoveries. Several research groups are developing diagnostic assays to detect mitochondrial transfer in patient samples, which could serve as predictive biomarkers for immunotherapy response. These tests might analyze circulating T cells for cancer-specific mtDNA mutations or measure mitochondrial function in tumor biopsies. If validated, such biomarkers could guide treatment decisions within the next few years. Meanwhile, pharmaceutical companies are beginning to invest in mitochondrial-targeted therapies for cancer, recognizing that metabolic reprogramming of immune cells represents an untapped therapeutic opportunity. The convergence of single-cell technologies, metabolomics, and advanced imaging is enabling researchers to map the tumor microenvironment with unprecedented resolution, revealing additional cell-cell interactions that cancer exploits. As we decode these mechanisms, the vision of truly personalized immunotherapy—tailored not just to the tumor's genetics but to the metabolic state of each patient's immune system—is coming into focus. The next decade will likely see the first clinical trials testing combinations of checkpoint inhibitors with metabolic modulators, potentially transforming outcomes for patients who currently have no effective options.
Conclusion
The revelation that cancer cells sabotage immune responses through mitochondrial transfer fundamentally changes how we think about tumor immunology. This isn't just another immune checkpoint or suppressive cytokine—it's a direct metabolic attack on the cells meant to destroy the tumor. What makes this discovery so significant is that it connects multiple threads in cancer research: the metabolic reprogramming of tumors, the phenomenon of T cell exhaustion, and the variable responses to immunotherapy. By understanding how cancer cells weaponize their own damaged mitochondria, researchers have identified new vulnerabilities that could be exploited therapeutically. The path forward involves not only blocking this transfer mechanism but also repairing the metabolic damage it causes and preventing the exhaustion pathways it triggers. As clinical trials begin testing these concepts, there's genuine hope that we can restore immune function in patients whose T cells have been compromised, extending the benefits of immunotherapy to many more people. The tumor microenvironment remains a battlefield, but we're finally learning the enemy's tactics—and developing countermeasures that could turn the tide in this cellular war.
References
- Ikeda H, Kawase K, Nishi T, et al. (2025). Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature. 638:225-236. PMID: 37089525
- Murmu A, Győrffy B (2025). Targeting the HMGB1-IL32 pathway to alleviate T cell exhaustion in epithelial ovarian cancer. Geroscience. 2025 Nov 15 (Online ahead of print). PMID: 41240232
- Passardi A, Sullo FG, Bittoni A, et al. (2025). CombiCoR-Vax trial: study protocol for a phase II, single-arm, multicenter trial of sequential pembrolizumab plus dendritic cell vaccine followed by trifluridine/tipiracil and bevacizumab in refractory microsatellite-stable metastatic colorectal cancer. BMC Cancer. 2025 Nov 22 (Online ahead of print). PMID: 41275134
- Behrens M, Comabella M, Lünemann JD (2024). EBV-specific T-cell immunity: relevance for multiple sclerosis. Frontiers in Immunology. 2024 Dec 24;15:1509927. PMID: 39776919
- Li M, Yin H, Jin Y, et al. (2025). A Real-World Study of Resectable NSCLC Following Neoadjuvant Immunotherapy: Should Postoperative Adjuvant Immunotherapy be Recommended?. Thoracic Cancer. 2025 Dec;16(23):e70195. PMID: 41334830
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026