Litifilimab: A Promising Anti-BDCA2 Antibody for Lupus Treatment
Quick Facts About Litifilimab
What is Litifilimab?
Litifilimab is a monoclonal antibody developed by Biogen targeting BDCA2, a receptor on plasmacytoid dendritic cells (pDCs), to regulate immune response in lupus.
What is the mechanism of action of Litifilimab?
Litifilimab inhibits BDCA2, reducing inflammatory cytokine production and modulating the immune system, potentially benefiting lupus patients.
What are the clinical applications of Litifilimab?
Litifilimab is being investigated for systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE), with Phase 3 trials ongoing.
1.) Understanding Litifilimab
Developed by Biogen, Litifilimab has shown promising results in early clinical trials, demonstrating its potential as a targeted therapy for lupus. Lupus, particularly systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE), is characterized by aberrant immune activation, with plasmacytoid dendritic cells (pDCs) playing a pivotal role in disease pathogenesis. These cells produce large amounts of type I interferons (IFN-I) and other pro-inflammatory cytokines, which contribute to the inflammatory cascade that drives lupus symptoms and tissue damage. By specifically binding to BDCA2, Litifilimab downregulates pDC activation, leading to a significant reduction in IFN-I signaling and cytokine release. This mechanism offers a novel approach to modulating immune responses in lupus patients.
In Phase 2 clinical trials, Litifilimab demonstrated encouraging efficacy in reducing skin disease activity in CLE patients and improving disease markers in SLE. The LILAC study, a randomized, placebo-controlled trial, provided key evidence of its therapeutic potential, showing significant improvements in clinical endpoints, including reductions in skin inflammation and systemic disease activity. The drug was also well tolerated, with a safety profile comparable to other biologic therapies in autoimmune diseases.
Ongoing Phase 3 trials aim to further assess Litifilimab’s long-term efficacy, safety, and tolerability across diverse patient populations. These studies will provide crucial insights into its potential as a disease-modifying treatment for lupus. If successful, Litifilimab could represent a breakthrough therapy, offering a more targeted and potentially safer alternative to conventional immunosuppressive treatments currently used in lupus management.
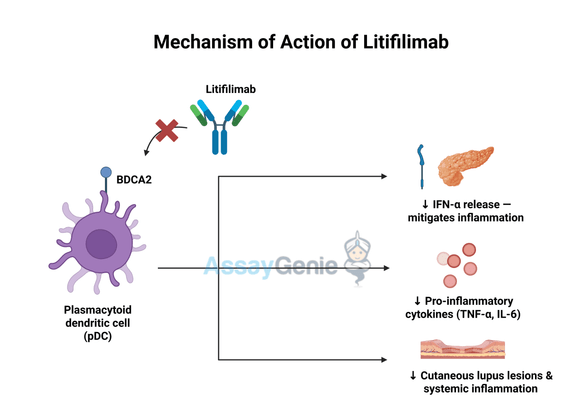
2.) Mechanism of Action of Litifilimab
Litifilimab exerts its therapeutic effects by binding to BDCA2, a type II C-type lectin receptor found exclusively on plasmacytoid dendritic cells (pDCs). BDCA2 plays a crucial role in regulating immune responses by modulatingc and inflammatory cytokine secretion. pDCs are central players in autoimmune diseases like systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE), where excessive type I interferon signaling drives disease progression and tissue damage.
By specifically targeting BDCA2, Litifilimab disrupts pDC-mediated immune activation through multiple mechanisms:
- Suppressing IFN-α production: Lupus patients often exhibit elevated levels of IFN-α, which perpetuate inflammation and autoimmunity. Litifilimab significantly reduces IFN-α release, mitigating the downstream inflammatory cascade.
- Inhibiting inflammatory pathway activation: The drug blocks pDC-driven secretion of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are implicated in immune dysregulation and tissue destruction.
- Potentially reducing lupus-related skin lesions and systemic inflammation: By limiting immune cell activation and cytokine release, Litifilimab may help alleviate cutaneous manifestations of lupus and prevent exacerbation of systemic symptoms.
Preclinical studies and early-phase clinical trials have demonstrated Litifilimab’s ability to downregulate pathogenic immune responses, showcasing its potential as a targeted therapy for lupus. Ongoing research aims to further elucidate its long-term efficacy and safety, potentially positioning Litifilimab as a breakthrough treatment for autoimmune diseases.
3.) Clinical Applications of Litifilimab
Litifilimab is currently under investigation for its role in treating:
1. Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by widespread systemic inflammation, leading to multi-organ involvement, including the skin, joints, kidneys, and cardiovascular system. A key driver of SLE pathogenesis is excessive type I interferon (IFN-I) signaling, which contributes to immune dysregulation and sustained inflammation. Litifilimab, by selectively targeting BDCA2 on plasmacytoid dendritic cells (pDCs), effectively suppresses IFN-α production and dampens inflammatory cytokine release.
In clinical trials, Litifilimab has demonstrated promising efficacy in reducing SLE disease activity, as measured by improvements in composite disease activity scores. It has been particularly effective in patients with high baseline interferon signatures, a subgroup known to exhibit more severe disease manifestations. Additionally, its ability to selectively modulate immune responses without broadly suppressing adaptive immunity offers a potentially safer alternative to conventional immunosuppressants and corticosteroids, which are associated with significant side effects.
2. Cutaneous Lupus Erythematosus (CLE)
Cutaneous lupus erythematosus (CLE) is a subtype of lupus that primarily affects the skin, leading to disfiguring lesions, photosensitivity, and chronic inflammation. Many CLE patients experience limited treatment options, as existing therapies often provide incomplete relief or have significant adverse effects.
Litifilimab has shown promising results in Phase 2 clinical trials, significantly reducing skin lesion severity and inflammation. By modulating pDC activity and suppressing IFN-α-driven skin pathology, it provides a targeted therapeutic approach for CLE.
Recent studies highlight its potential to address unmet medical needs in lupus management, with ongoing Phase 3 trials evaluating its long-term efficacy, safety, and potential as a first-in-class targeted therapy for both SLE and CLE.

4.)Exploring Biosimilars for Litifilimab
As research advances, biosimilars of Litifilimab are emerging as valuable tools for studying BDCA2-targeted therapies.
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an already approved reference biologic, with no clinically meaningful differences in efficacy, safety, or immunogenicity. Biosimilars play a crucial role in research by providing cost-effective alternatives for studying novel therapeutics.
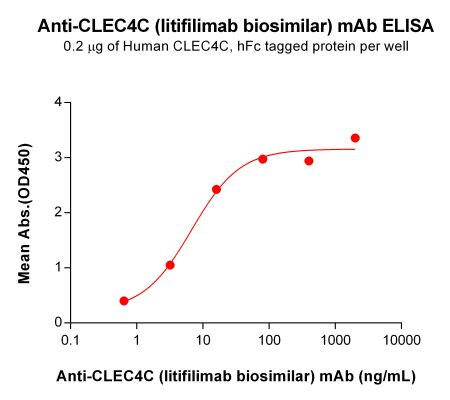
| Litifilimab (Anti-CLEC4C) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CLEC4C |
| Reactivity: | Human |
How Litifilimab Biosimilar Compares to Litifilimab
Biosimilars of Litifilimab retain the core mechanism of BDCA2 inhibition while being optimized for research applications. They offer:
- High similarity in structure and function.
- Accessibility for in vitro and in vivo research.
- Cost-efficient options for exploring BDCA2-targeted interventions
Advancing Research on Litifilimab
Litifilimab biosimilars provide researchers with a powerful tool to investigate:
- BDCA2 pathway mechanisms in autoimmune diseases.
- Potential combination therapies for lupus.
- Long-term immune modulation effects.
Research Use Only Disclaimer:
Litifilimab biosimilars are intended for research purposes only and are not approved for clinical use.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026