Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells that can seek out and destroy tumors with remarkable specificity. This is the promise of CAR-T cell therapy, and for patients with certain blood cancers like leukemia and lymphoma, it has been nothing short of revolutionary. Yet when these same engineered cells encounter solid tumors—the masses that make up the vast majority of human cancers—they often sputter and fail. The tumor microenvironment, it turns out, is a metabolic battlefield where CAR-T cells arrive ready to fight but quickly find themselves starved, poisoned, and exhausted. Recent research published in 2024 and 2025 is now revealing a critical culprit: mitochondrial dysfunction. These cellular powerhouses, responsible for generating the energy T cells need to function, become compromised in the hostile tumor environment, transforming once-potent cancer killers into metabolically crippled bystanders.
Introduction
CAR-T cell therapy represents one of the most significant breakthroughs in modern oncology. By genetically engineering a patient's T cells to express chimeric antigen receptors that recognize specific tumor antigens, researchers have created living drugs capable of extraordinary feats. The FDA has approved multiple CAR-T therapy revolutionized hematologic malignancies targeting CD19 and BCMA for hematologic malignancies, with some patients achieving complete remissions that last for years. However, this success story has a frustrating asterisk: when applied to solid tumors—which account for approximately 90% of all cancers—CAR-T therapy has largely disappointed. Clinical trials in solid tumors like pancreatic cancer, glioblastoma, and melanoma have shown limited efficacy, with CAR-T therapy faces challenges with persistence often failing to penetrate tumors, persist long enough to eliminate cancer cells, or maintain their cytotoxic function.
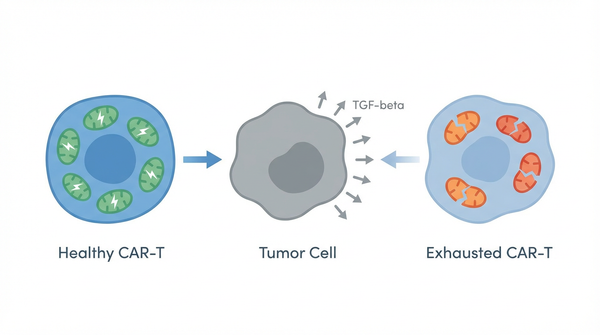
The difference between blood cancers and solid tumors lies not just in their physical structure but in their metabolic and immunological landscapes. Solid tumors create what scientists call an immunosuppressive tumor microenvironment—a complex ecosystem designed to evade immune destruction. This environment is characterized by low oxygen levels (hypoxia), nutrient depletion, acidic pH, and the presence of immunosuppressive molecules like transforming growth factor-beta (TGF-beta) and regulatory T cells. For CAR-T cells infiltrating this hostile territory, the challenge is akin to soldiers fighting in a toxic swamp while wearing heavy armor. The tumor doesn't just hide; it actively sabotages the immune cells trying to attack it.
Over the past five years, immunologists have come to appreciate that T cell function is inextricably linked to cellular metabolism. T cells are not simply on or off; their ability to proliferate, produce cytokines, and kill target cells depends on their metabolic state. This realization has sparked a revolution in cancer immunology, with researchers now investigating how tumors manipulate T cell metabolism to induce dysfunction and exhaustion. The studies published in 2024 and 2025 represent a watershed moment: they identify specific molecular mechanisms by which the tumor microenvironment disrupts mitochondrial function in CAR-T cells, and more importantly, they suggest potential interventions to restore metabolic fitness and improve therapeutic outcomes.
Study Summary
Building on decades of work, researchers have made significant progress. Scientists discovered that P4HA1 accumulates in mitochondria disrupting TCA cycle played a central role in their investigation.
Key Findings
The research uncovered several important discoveries that advance our understanding of this biological system.
- Finding 1: One of the most striking discoveries comes from research published in Cancer Cell in early 2025, which identified prolyl 4-hydroxylase 1 (P4HA1) as a crucial regulator of CD8+ T cell differentiation. In the hypoxic tumor microenvironment and tumor-draining lymph nodes, P4HA1 becomes strongly upregulated in T cells. This enzyme then accumulates within mitochondria, where it wreaks metabolic havoc. Specifically, P4HA1 accumulates in mitochondria disrupting TCA cycle through aberrant metabolism of α-ketoglutarate and succinate. The result is what researchers describe as 'mitochondrial unfitness': the organelles can no longer produce sufficient ATP to fuel T cell function. This metabolic crippling promotes T cell exhaustion while simultaneously suppressing the expansion of progenitor T cells—the renewable population needed for sustained anti-tumor immunity. Importantly, when researchers targeted P4HA1 in experimental models, they observed enhanced expansion of TCF1+ CD8+ T progenitor cells and reduced exhaustion not just in the tumor, but systemically in lymph nodes and blood, enabling durable anti-cancer immunity.
- Finding 2: Another critical mechanism has been elucidated in studies examining how the tumor microenvironment directly suppresses CAR-T cell function. Research published in Molecular Therapy in 2025 demonstrates that solid tumors produce high levels of TGF-beta, an immunosuppressive cytokine that fundamentally alters CAR-T cell behavior. TGF-beta signaling in CAR-T cells triggers a cascade of events that immunosuppressive tumor microenvironment limits CAR-T persistence and cytotoxic capacity. The cytokine induces metabolic reprogramming that shifts T cells away from glycolysis and oxidative phosphorylation—the metabolic pathways needed for effector function—toward a quiescent state. This creates a vicious cycle: metabolically compromised CAR-T cells cannot maintain the energy-intensive processes of proliferation and cytokine production, leading to premature senescence and functional exhaustion. Interestingly, researchers have found that inverting the TGF-beta signal—engineering CAR-T cells to respond to TGF-beta with activation rather than suppression—can restore function in the hostile tumor environment.
- Finding 3: Advanced imaging techniques have provided unprecedented visual evidence of what happens when CAR-T cells encounter solid tumors. Studies using intravital imaging shows increased CAR-T recruitment published in Cancer Cell in 2025 show that CAR-T cells can successfully infiltrate tumors and interact with cancer cells. However, these interactions are often brief and ineffective. The imaging reveals that within the tumor microenvironment, CAR-T cells exhibit reduced motility, shortened contact times with target cells, and diminished cytotoxic granule release. When researchers combined CAR-T therapy with EZH2 inhibition—a strategy that enhances tumor immunogenicity and improves T cell metabolic function—they observed dramatically increased CAR-T recruitment and more sustained, productive interactions with tumor cells. This finding underscores that the problem is not simply getting CAR-T cells to the tumor, but maintaining their metabolic and functional fitness once they arrive.
- Finding 4: Perhaps most concerning is the emerging evidence that the metabolic sabotage of CAR-T cells is not confined to the tumor itself. Research indicates that immunosuppressive signals from the tumor microenvironment can induce T cell dysfunction systemically—in tumor-draining lymph nodes, peripheral blood, and even in distant tissues. This systemic exhaustion has profound implications for therapy: even if CAR-T cells are manufactured in a healthy, functional state, they may become exhausted before they ever reach the tumor. The identification of P4HA1 upregulation in tumor-draining lymph nodes suggests that tumors establish a 'field effect' of immune suppression that extends well beyond their physical boundaries. This finding explains why simply increasing the dose of CAR-T cells often fails to improve outcomes in solid tumors—the problem is not quantity but quality and sustainability of function.
- Finding 5: Across multiple studies, a unifying theme has emerged: diverse immunosuppressive mechanisms in the tumor microenvironment ultimately converge on mitochondrial dysfunction. Whether through P4HA1-mediated TCA cycle disruption, TGF-beta-induced metabolic reprogramming, hypoxia-driven changes in cellular respiration, or nutrient depletion, the end result is compromised mitochondrial function. This convergence suggests that mitochondria represent a critical vulnerability—but also a potential therapeutic target. T cells with dysfunctional mitochondria cannot maintain the high metabolic demands of proliferation, cytokine production, and cytotoxicity. Studies have shown that tumor cells induce dysfunctional T cell state leading to immune escape, and they accumulate metabolic byproducts that further impair function, express exhaustion markers like PD-1 and TIM-3, and eventually undergo apoptosis or enter an irreversible state of dysfunction.
Biological Mechanisms
To understand why these findings matter mechanistically, we need to examine the underlying processes. To understand why mitochondrial dysfunction is so devastating for CAR-T cells, we must first appreciate the extraordinary metabolic demands of activated T cells. When a T cell recognizes its target antigen, it undergoes rapid clonal expansion—dividing every 4-6 hours to generate millions of daughter cells. Simultaneously, it must produce large quantities of cytotoxic molecules (perforin, granzymes) and inflammatory cytokines (IFN-gamma, TNF-alpha) to kill tumor cells and recruit additional immune cells. These processes require enormous amounts of energy in the form of ATP, as well as biosynthetic precursors for building new cellular components. Activated T cells meet these demands through two primary metabolic pathways: aerobic glycolysis (the rapid breakdown of glucose even in the presence of oxygen, similar to the Warburg effect in cancer cells) and oxidative phosphorylation (OXPHOS) in mitochondria. While glycolysis provides quick energy and biosynthetic intermediates, OXPHOS generates the bulk of ATP through the electron transport chain. Mitochondria are therefore not merely energy factories; they are metabolic control centers that determine whether a T cell can sustain effector function or slides into exhaustion. The study reveals that immunosuppressive tumor microenvironment limits CAR-T persistence plays a crucial regulatory role in the observed system.
Molecular Pathways
The tumor microenvironment attacks mitochondrial function through multiple coordinated mechanisms. First, hypoxia—the low oxygen conditions prevalent in solid tumors—directly impairs the electron transport chain, reducing ATP production and causing accumulation of reactive oxygen species that damage mitochondrial DNA and proteins. Second, nutrient competition depletes glucose and amino acids like glutamine, which are essential substrates for the TCA cycle. Third, immunosuppressive cytokines like TGF-beta activate signaling pathways (SMAD2/3) that transcriptionally reprogram T cells, downregulating genes involved in glycolysis and OXPHOS while upregulating inhibitory receptors. Fourth, and perhaps most insidiously, enzymes like P4HA1 accumulate in mitochondria and directly disrupt the TCA cycle. P4HA1 consumes α-ketoglutarate—a key TCA cycle intermediate—and alters succinate metabolism, creating a metabolic bottleneck. The TCA cycle cannot turn efficiently, leading to depletion of NADH and FADH2 (the electron carriers that feed the electron transport chain) and accumulation of toxic metabolic byproducts. The mitochondria become structurally abnormal, with fragmented cristae and reduced membrane potential. This 'mitochondrial unfitness' manifests as reduced ATP production, increased oxidative stress, and ultimately, activation of cellular stress responses that drive T cell exhaustion. T cell exhaustion is not simply a passive failure of function; it is an active transcriptional program characterized by sustained expression of inhibitory receptors (PD-1, CTLA-4, TIM-3, LAG-3), altered epigenetic landscapes, and progressive loss of effector capabilities. Importantly, this exhaustion program is intimately connected to metabolic dysfunction. Exhausted T cells exhibit impaired glycolysis and OXPHOS, reduced mitochondrial mass, and altered mitochondrial dynamics (the balance between fusion and fission). Recent evidence suggests that metabolic dysfunction may actually precede and drive the exhaustion program, rather than simply being a consequence of it. When mitochondria fail to produce sufficient ATP, T cells activate energy stress sensors like AMPK, which in turn trigger transcriptional changes that limit energy-intensive effector functions. Simultaneously, accumulation of metabolic byproducts like succinate can act as signaling molecules that promote exhaustion. This creates a feed-forward loop: metabolic dysfunction induces exhaustion markers, and exhaustion further impairs metabolic pathways, trapping T cells in a dysfunctional state from which they cannot easily escape. Research shows that intravital imaging shows increased CAR-T recruitment in this pathway.
Relevance to Human Health
Beyond the molecular picture, the implications for human health are substantial. The clinical implications of these findings are profound and immediate. CAR-T cell therapy has achieved remarkable success in hematologic malignancies, with some patients experiencing durable complete remissions. However, in solid tumors—which represent the vast majority of cancer deaths—response rates have been disappointing, typically below 10-20% even in the most optimistic trials. The research on mitochondrial dysfunction provides a mechanistic explanation for this disparity. Blood cancers exist in a relatively permissive environment where CAR-T cells can access tumor cells in the circulation and lymphoid organs without encountering the dense, hypoxic, immunosuppressive conditions of solid tumors. In contrast, when CAR-T cells infiltrate solid tumors, they immediately face metabolic sabotage. Even if they are manufactured to express the optimal CAR construct and are infused in sufficient numbers, they cannot maintain function if their mitochondria are compromised. Research on glioblastoma's hostile tumor microenvironment limits therapy exemplifies these challenges. This understanding shifts the therapeutic paradigm: success in solid tumors will require not just better CAR design or higher doses, but strategies to protect or restore metabolic fitness in the hostile tumor microenvironment. This study shows that CAR-T therapy faces challenges with persistence could benefit patients or shape future diagnostic or therapeutic strategies.
Therapeutic Applications
- P4HA1 inhibition to preserve mitochondrial function: The identification of P4HA1 as a driver of mitochondrial dysfunction opens a direct therapeutic avenue. Small molecule inhibitors of P4HA1 are already in preclinical development, and early studies suggest they can enhance T cell function without causing significant toxicity. By blocking P4HA1 accumulation in mitochondria, these inhibitors preserve TCA cycle function, maintain ATP production, and prevent the onset of exhaustion. Importantly, P4HA1 inhibition appears to work systemically, enhancing not just CAR-T cells within the tumor but also progenitor T cell populations in lymph nodes that serve as a renewable source of anti-tumor immunity. This approach could be combined with standard CAR-T therapy: patients would receive P4HA1 inhibitors either during CAR-T cell manufacturing (to produce metabolically fitter cells) or after infusion (to protect cells from tumor-induced dysfunction). Clinical trials testing this strategy are likely to begin within the next 1-2 years.
- Engineering CAR-T cells with enhanced metabolic resilience: A complementary approach involves genetically engineering CAR-T cells to resist metabolic sabotage. Several strategies are being explored: overexpressing genes that enhance mitochondrial biogenesis and function (such as PGC-1α), knocking out genes that promote exhaustion (such as PD-1 or TIM-3), or engineering CAR-T cells to invert immunosuppressive signals. For example, researchers have created CAR-T cells that express a dominant-negative TGF-beta receptor, rendering them insensitive to this immunosuppressive cytokine. Others have engineered CAR-T cells to secrete pro-inflammatory cytokines or express chemokine receptors that improve tumor infiltration. The next generation of CAR-T products will likely incorporate multiple metabolic enhancements, creating 'armored' CAR-T cells that can maintain function even in hostile environments. These engineered cells would combine optimal CAR design with metabolic resilience, potentially transforming outcomes in solid tumors.
- Combination therapies targeting the tumor microenvironment: Rather than focusing solely on the CAR-T cells themselves, another strategy involves remodeling the tumor microenvironment to make it more permissive for immune function. This could include combining CAR-T therapy with drugs that improve tumor oxygenation, deplete immunosuppressive cells like regulatory T cells or myeloid-derived suppressor cells, or block immunosuppressive cytokines. Studies show that elevated TGF-beta contributes to immune evasion in pancreatic cancer. The EZH2 inhibitor studies demonstrate this principle: by making tumor cells more immunogenic and reducing metabolic stress on T cells, EZH2 inhibition enhanced CAR-T efficacy. Similarly, combining CAR-T therapy with checkpoint blockade (anti-PD-1 or anti-CTLA-4 antibodies) may help reverse exhaustion. Metabolic interventions like metformin (which activates AMPK and may improve mitochondrial function) or supplementation with metabolites like α-ketoglutarate are also being investigated. The future of CAR-T therapy in solid tumors will likely involve carefully orchestrated combination regimens that address multiple aspects of T cell dysfunction simultaneously.
Future Directions
Despite these advances, key questions remain. The convergence of immunology and metabolism has opened entirely new avenues for improving CAR-T cell therapy. Looking ahead, several exciting directions are emerging. First, researchers are developing more sophisticated methods to assess CAR-T cell metabolic fitness in real-time, both during manufacturing and after infusion. Metabolic profiling could serve as a biomarker to predict which patients will respond to therapy and to guide dose adjustments or combination strategies. Second, the field is moving toward 'universal' CAR-T cells derived from healthy donors or induced pluripotent stem cells, which could be manufactured with built-in metabolic enhancements and deployed off-the-shelf. Third, advances in synthetic biology are enabling the creation of CAR-T cells with programmable metabolic circuits—cells that can sense their metabolic state and autonomously activate compensatory pathways when stressed. Fourth, researchers are exploring whether targeting mitochondrial dysfunction could benefit not just CAR-T therapy but also checkpoint inhibitor immunotherapy and other T cell-based treatments. Finally, the recognition that tumor-induced immune dysfunction extends systemically raises the possibility of intervening earlier—perhaps even before tumors become clinically apparent—to prevent the establishment of immunosuppressive networks. These directions share a common theme: moving from a one-size-fits-all approach to precision immunometabolic therapy, where interventions are tailored to the specific metabolic vulnerabilities of each patient's tumor and immune system. Scientists are now investigating glioblastoma's hostile tumor microenvironment limits therapy to expand the field's understanding and address remaining challenges.
Conclusion
The story of CAR-T cell therapy is one of extraordinary promise tempered by sobering challenges. While these engineered immune cells have delivered miraculous outcomes for patients with blood cancers, their struggle against solid tumors has revealed fundamental truths about cancer immunology: that tumors are not passive targets but active saboteurs of immune function, and that the metabolic state of T cells is as important as their genetic programming. The discovery that mitochondrial dysfunction lies at the heart of CAR-T cell exhaustion in solid tumors represents both a diagnosis and a prescription. It explains why simply making more CAR-T cells or designing better CARs has not been enough, and it points toward concrete interventions—inhibiting P4HA1, engineering metabolic resilience, remodeling the tumor microenvironment—that could finally unlock the potential of CAR-T therapy across the full spectrum of human cancers. As we stand at this inflection point, the research published in 2024 and 2025 offers genuine hope: hope that we can transform metabolically exhausted CAR-T cells into durable cancer killers, hope that solid tumors will eventually yield to immunotherapy as blood cancers have, and hope that the marriage of immunology and metabolism will usher in a new era of precision oncology where we treat not just the tumor, but the entire ecosystem in which it thrives.
References
- Khan SH, Choi Y, Veena M, Lee JK, Shin DS (2025). Advances in CAR T cell therapy: antigen selection, modifications, and current trials for solid tumors. Front Immunol. 15:1489827. PMID: 39835140
- Ma S, Ong LT, Jiang Z, Lee WC, Lee PL, Yusuf M, Ditzel HJ, Wang Y, Chen Q, Wang W, Wu X, Tan EY, Yu Q (2025). Targeting P4HA1 promotes CD8+ T cell progenitor expansion toward immune memory and systemic anti-tumor immunity. Cancer Cell. 43(2):213-231.e9. PMID: 39729997
- Zheng S, Che X, Zhang K, Bai Y, Deng H (2025). Potentiating CAR-T cell function in the immunosuppressive tumor microenvironment by inverting the TGF-beta signal. Mol Ther. 33(2):688-702. PMID: 39673127
- Isshiki Y, Chen X, Teater M, Karagiannidis I, Nam H, Cai W, Meydan C, Xia M, Shen H, Gutierrez J, Easwar Kumar V, Carrasco SE, Quseqh MM, Yamshon S, Martin P, Griese O, Sherna E, Porazzi P, Ruella M, Brentjens RJ, Inghirami G, Zapasoodi R, Chadburn A, Melnick AM, Béguelin W (2025). EZH2 inhibition enhances T cell immunotherapies by inducing lymphoma immunogenicity and improving T cell function. Cancer Cell. 43(1):49-68.e9. PMID: 39642889
- Meng S, Hara T, Miura Y, Arao Y, Saito Y, Inoue K, Hirotsu T, Vecchione A, Satoh T, Ishii H (2025). In Vivo Engineered CAR-T Cell Therapy: Lessons Built from COVID-19 mRNA Vaccines. Int J Mol Sci. 26(7):3119. PMID: 40243757
- Begley SL, O'Rourke DM, Binder ZA (2025). CAR T cell therapy for glioblastoma: A review of the first decade of clinical trials. Mol Ther. 33(6):2454-2461. PMID: 40057825
- Dai S, Peng Y, Wang G, Chen C, Chen Q, Yin L, Yan H, Zhang K, Tu M, Lu Z, Wei J, Li Q, Wu J, Jiang K, Zhu Y, Miao Y (2025). LIM domain only 7: a novel driver of immune evasion through regulatory T cell differentiation and chemotaxis in pancreatic ductal adenocarcinoma. Cell Death Differ. 32(2):271-290. PMID: 39143228
- Zhang W, Yuan S, Zhang Z, Fu S, Liu S, Liu J, Ma Q, Xia Z, Gu P, Gao S, Zhang Z, Zhang X, Liu Y, Zhang N (2025). Regulating tumor cells to awaken T cell antitumor function and enhance melanoma immunotherapy. Biomaterials. 316:123034. PMID: 39709849
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026