Palivizumab (Synagis®) Quantitative ELISA
- SKU:
- HUMB00072
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Palivizumab (Synagis®)
- Research Area:
- Anti-Inflammatory
Description
Palivizumab (Synagis®) Quantitative ELISA Kit (HUMB00072)
The Assay Genie Palivizumab Qualitative ELISA has been developed for determination of quantitative antibodies to Palivuzumab in serum and plasma samples.
Palivizumab (Synagis®) Quantitative ELISA Kit - Test Principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the drug Palivuzumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to Palivuzumab antibodies captured by the drug Palivuzumab on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of Palivuzumab antibodies in the sample or standard. Results of samples can be determined directly using the standard curve.
Palivizumab (Synagis®) Quantitative ELISA - Product Information
| Information | Description |
| Application | Determination of quantitative antibodies to Palivuzumab in serum and plasma samples. |
| Required Volume (µl) | 10 |
| Total Time (min) | 140 |
| Sample Typle | Serum, plasma |
| Number of Assays | 96 |
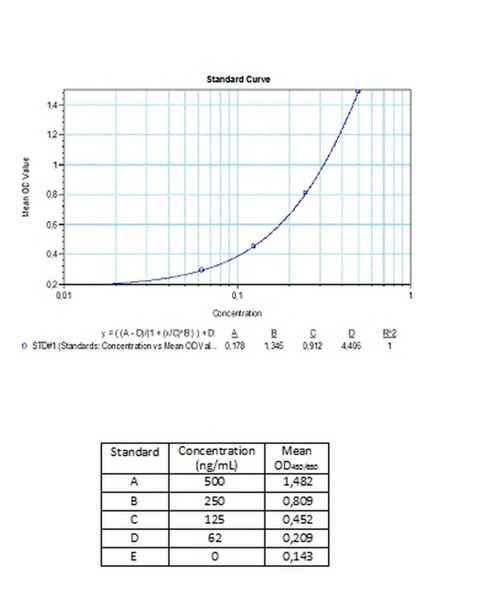
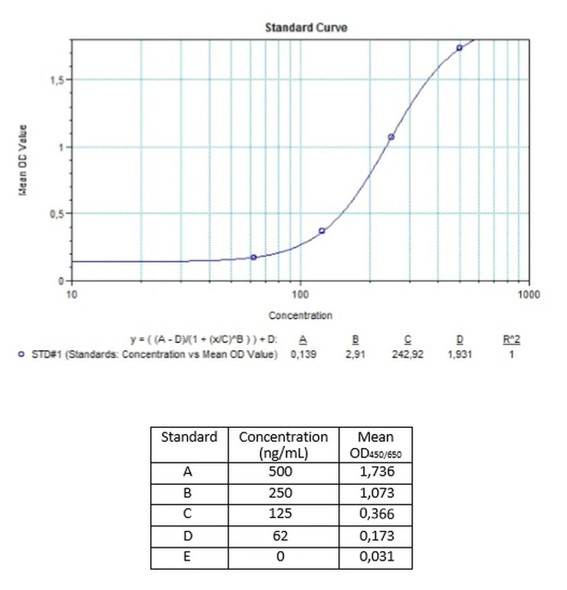
| Detection Limit (ng/mL) | 62,5 |
| Spike recovery (%) | 85-115 |
| Shelf Life (year) | 1 |
Palivizumab (Synagis®) Quantitative ELISA - General Information
The humanised monoclonal antibody palivizumab has been developed for prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in infants at high risk; RSV is the most common cause of lower respiratory tract infections in infants. Palivizumab specifically inhibits an epitope at the A antigenic site of the F protein of RSV subtypes A and B. Synagis® (palivizumab) is a humanized monoclonal antibody (IgG1k) produced by recombinant DNA technology, directed to an epitope in the A antigenic site of the F protein of respiratory syncytial virus (RSV). Palivizumab is a composite of human (95%) and murine (5%) antibody sequences. The human heavy chain sequence was derived from the constant domains of human IgG1 and the variable framework regions of the VH genes Cor and Cess. The human light chain sequence was derived from the constant domain of Ck and the variable framework regions of the VL gene K104 with Jk -4.
Lower respiratory tract disease caused by respiratory syncytial virus (RSV) accounts for more than 125,000 pediatric hospitalizations and approximately 6.3 deaths per 100,000 person-years among children up to 4 years of age in the United States. Children more likely to have increased morbidity and mortality rates as the result of complications of RSV infection include those with prematurity, chronic lung disease, or congenital heart disease (CHD). In children with cardiac abnormalities, one early study showed a 37% fatality rate for RSV-infected, hospitalized infants with CHD. More recent studies suggest that among infants with CHD who are hospitalized with RSV disease, 33% will require treatment in a pediatric intensive care unit (ICU), and 2.5% to 3.4% die of complications of the RSV infection. Infants with pulmonary hypertension are at greatest risk from RSV infection. In addition, nosocomial RSV infection has a particularly adverse impact on infants and children who become infected in the immediate preoperative or postoperative period for heart surgery.
Therapeutic drug monitoring (TDM) is the clinical practice of measuring specific drugs at designated intervals to maintain a constant concentration in a patient's bloodstream, thereby optimizing individual dosage regimens. The indications for drug monitoring include efficacy, compliance, drug-drug interactions, toxicity avoidance, and therapy cessation monitoring. Additionally, TDM can help to identify problems with medication compliance among noncompliant patient cases.
Biologic medicinal products (biologics) have transformed treatment landscapes worldwide for patients with haematological or solid malignancies with the 21st century. Today, as data exclusivity periods of first wave biologics approach expiration/have expired, several biosimilar products (i.e.,biologics that are considered to be similar in terms of quality, safety and efficacy to an approved 'reference' biologic) are being developed or have already been approved for human use.
Like all biologics, biosimilars are structurally complex proteins that are typically manufactured using genetically engineered animal, bacterial or plant cell culture systems. As a consequence of this molecular complexity and the proprietary nature of the manufacturing process, which will inevitably result in the use of different host cell lines and expression systems as well as related differences in manufacturing conditions, it is not possible to manufacture exact copies of a reference biologic.
When administered to patients, all therapeutic proteins have the potential to induce an unwanted immune response (i.e.,to stimulate the formation of antidrug antibodies [ADAs]). The impact of immune responses can range from no apparent effect to changes in pharmacokinetics, loss of effect and seriousadverse events. Furthermore, the immunogenicity profile of a biologic can be significantly altered by even small differences in its manufacturing process that are accompanied by a change in product attributes, as well as differences in dosing schedules, administration routes or patient populations.
Palivizumab (Synagis®) Qualitative ELISA - Kit Contents
| Size | Kit Contents |
| 1x12x8 |
Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
| 1,0 mL (each) |
Standards A-E (100x) Standard A: 500 ng/mL Standard B: 250 ng/mL Standard C: 125 ng/mL Standard D: 62,5 ng/mL Standard E: 0 ng/mL Ready to use. Used for the standard curve. Contains antibodies to Palivuzumab, human serum and stabilizer, <0,1% NaN3. |
| 1 mL (each) |
Control low and high levels Ready to use. Contains human serum and stabilizer, <0,1% NaN3. Control concentrations are given in "Quality control certificate" |
| 1 x 50 mL |
Assay buffer Ready to use. Blue coloured. Contains proteins, <0,1 % NaN3 |
| 1x12 mL |
Horse radish peroxidase conjugated probe Ready to use. Red coloured. Contains HRP conjugated probe, stabilizer and preservatives. |
| 1x12 mL |
TMB substrate solution. Ready to use. Contains 3,3′,5,5′- Tetramethylbenzidine (TMB). |
| 1x12mL |
TMB stop solution. Ready to use. 1N HCl |
| 1x50mL |
Wash buffer (20x). Prepared concentrated (20x) and should be diluted with the dilution rate given in the “Pretest setup instructions” before the test. Contains buffer with tween 20. |
| 2x1 |
Adhesive Foil. For covering microtiter plate during incubation |
Palivizumab (Synagis®) Qualitative ELISA - Kit Protocol
| 1 |
Pipette 100 µL of each "Standards", "Low level control", "High
level control" and diluted samples into the respective wells of
microtiter plate.
Wells A1: Standard A B1: Standard B C1: Standard C D1: Standard D E1: Standard E F1: Low level control G1: High level control H1 and on: Samples *It is advised to run more than one "Standard E (negative control)" samples for qualitative assay. Negative control studies can be duplicated or triplicated in order to take the mean value. |
| 2 | Cover the plate with adhesive foil Briefly mix contents by gently shaking the plate Incubate 30 minutes at room temperature (18-25°C) |
| 3 | Remove adhesive foil. Discard incubation solution. Wash plate three times each with 300 µL Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel |
| 4 | Pipette 100 µL Conjugate into each well |
| 5 | Cover the plate with adhesive foil. Incubate 30 minutes at room temperature (18-25°C) |
| 6 | Remove adhesive foil. Discard incubation solution. Wash plate three times each with 300 µL Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel |
| 7 | Pipette 100 µL Substrate into each well |
| 8 | Incubate 20 minutes without adhesive foil at room temperature (18-25°C) in the dark |
| 9 | Stop the substrate reaction by adding 100 µL Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 10 | Measure optical density with a photometer at OD 450nm with reference wavelength 650 nm (450/650 nm) within 30 minutes after pipetting the Stop Solution. |