Autophagy in Focus: Molecular Mechanisms and Functional Insights
Autophagy, a fundamental cellular process, plays an indispensable role in maintaining cellular homeostasis and ensuring the efficient recycling of cellular components. Through this process, cells can adapt to various stressors, ensuring their survival and contributing to overall organismal health. In this blog, we focus on the multifaceted world of autophagy, unraveling its molecular intricacies and understanding its critical significance in cellular physiology and disease prevention.
Table of Contents
Jump to a section:
Introduction to Autophagy
Autophagy, an intricate and conserved cellular phenomenon, stands as a sentinel process that profoundly influences cellular equilibrium, ensuring efficient waste disposal and functional rejuvenation. Derived from the Greek lexicons "auto" (self) and "phagy" (eating), autophagy succinctly encapsulates the mechanism by which cells orchestrate their own self-digestion. This orchestration, however, is far from a haphazard degradation; rather, it is a meticulously regulated process that enables cells to selectively degrade and recycle a range of cellular constituents, from misfolded proteins and defunct organelles to aged cytoplasmic components.
At its core, autophagy serves as an adaptive mechanism, a biological strategy for cells to counteract fluctuations in nutrient availability, cellular stress, and myriad other environmental perturbations. By cannibalizing its own components, a cell can secure essential biomolecules for vital functions even when external nutrient sources are scarce. This capacity to self-sustain in adversity renders autophagy a critical player in maintaining cellular vitality and ensuring functional integrity.
This evolutionary conserved process is enacted through a series of precisely choreographed stages. Central to these stages is the formation of specialized double-membraned vesicles termed autophagosomes. These autophagosomes encapsulate the cellular cargo earmarked for degradation, sequestering them from the rest of the cellular milieu. Subsequent fusion of autophagosomes with lysosomes - membrane-bound organelles rife with an array of hydrolytic enzymes - culminates in the degradation of the engulfed cargo. This orchestrated sequence, characterized by its spatial and temporal precision, ensures that autophagy is a tightly regulated and purpose-driven mechanism.
Beyond its role in nutrient recycling, autophagy intersects with an array of cellular functions and physiological contexts. From modulating cellular differentiation and immune responses to combating microbial infections and contributing to developmental processes, the influence of autophagy reverberates across a spectrum of biological scenarios. Furthermore, disruptions in autophagy have been implicated in a myriad of human maladies, including neurodegenerative disorders, cancer, and metabolic diseases. The ability of autophagy to safeguard cellular health makes it a subject of fervent scientific inquiry, with researchers delving into its molecular intricacies to glean insights that could hold therapeutic potential.
[FIGURE LEGEND]
Cellular Mechanisms of Autophagy
Autophagy, a conserved and vital process, plays a pivotal role in maintaining cellular homeostasis by selectively eliminating damaged organelles, aggregated proteins, and intracellular pathogens. At its core, autophagy involves a series of orchestrated events within the cell. Initiation begins with the formation of a double-membrane structure called the phagophore, which engulfs the targeted cargo. This phagophore matures into an autophagosome, encapsulating the cargo within its membranes. The autophagosome subsequently fuses with lysosomes, forming an autolysosome. Within the acidic environment of the autolysosome, lysosomal hydrolases degrade the cargo, releasing essential building blocks that can be recycled for biosynthetic processes. This dynamic process is tightly regulated by a network of autophagy-related genes (ATGs) and signaling pathways, ensuring precision and adaptability in response to various cellular stresses. A better understanding of these intricate cellular mechanisms provides insights into not only fundamental cellular biology but also potential therapeutic strategies for a myriad of diseases associated with autophagic dysregulation.
Types of Autophagy
The concept of autophagy extends beyond a uniform process, encompassing a spectrum of specialized mechanisms that cells employ to ensure optimal functionality. Among these, macroautophagy stands as the archetypal pathway, characterized by its orchestrated process of cargo encapsulation, autophagosome formation, and fusion with lysosomes. This mechanism plays a fundamental role in preserving cellular health by selectively removing damaged organelles, misfolded proteins, and intracellular invaders. Microautophagy, in contrast, takes a more direct approach, as lysosomes themselves protrude and engulf portions of the cytoplasm. This type of autophagy offers an immediate response to cellular stressors, efficiently recycling small portions of cellular material.
Chaperone-mediated autophagy (CMA) stands out for its exquisite specificity. It targets individual proteins containing a characteristic motif recognized by chaperone proteins. These chaperones escort target proteins to lysosomal membranes, facilitating their translocation into lysosomes for degradation. This precise mode of autophagy allows cells to maintain protein quality control and respond to changes in protein demand. On a broader scale, non-selective autophagy, known as bulk autophagy, indiscriminately engulfs portions of the cytoplasm, providing a means of clearing cellular debris and metabolites.
While these autophagic pathways may seem distinct, they intertwine in a synchronized dance to uphold cellular equilibrium. For instance, macroautophagy and microautophagy can collaborate when engulfing larger portions of the cytoplasm, while CMA and bulk autophagy may work in concert during periods of cellular stress. This intricate interplay highlights the adaptability of cells to diverse challenges and the essential nature of autophagy in cellular survival.
Types of Autophagy
Autophagy Pathway and Regualtion
The autophagy pathway represents a highly orchestrated series of molecular events that safeguard cellular health and adaptability. Central to this pathway are the autophagy-related genes (ATGs), a group of evolutionarily conserved genes responsible for controlling various stages of autophagy. Initiation of autophagy involves the ULK1 protein complex, which senses nutrient deprivation and triggers the formation of the phagophore, the precursor structure of the autophagosome. The class III phosphatidylinositol 3-kinase complex, or PI3KC3-C1, is pivotal in this process, promoting phagophore nucleation and expansion.
The regulation of autophagy is exquisitely orchestrated by cellular signaling pathways. The mechanistic target of rapamycin complex 1 (mTORC1), a master regulator of cell growth and metabolism, actively inhibits autophagy in nutrient-rich conditions. Conversely, during nutrient scarcity or stress, mTORC1 is inhibited, unleashing the initiation of autophagy. AMP-activated protein kinase (AMPK), a sensor of cellular energy levels, also participates in autophagy regulation by activating ULK1 and promoting autophagy initiation.
Furthermore, autophagy is intertwined with cellular stress responses, including the unfolded protein response (UPR) and hypoxia-inducible factor 1α (HIF-1α) pathways. These stress pathways can both induce and be induced by autophagy, creating a complex interplay that helps cells adapt to changing environmental conditions.
Autophagy Related ELISA Kits
Related Kits
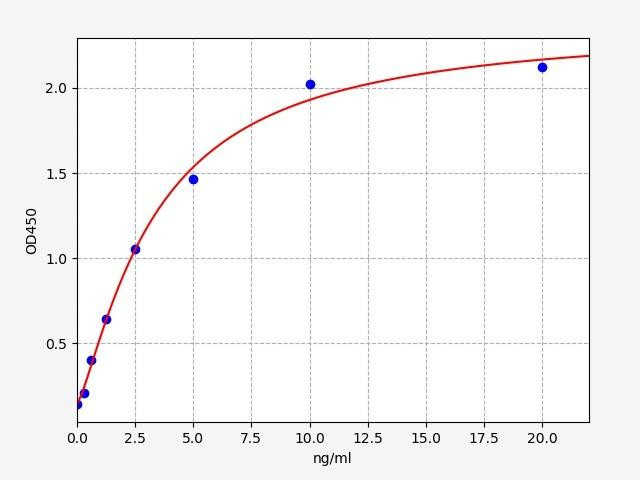
| Human ATG5 / Autophagy protein 5 ELISA Kit | |
|---|---|
| Sensitivity | 0.188ng/ml |
| Range | 0.313-20ng/ml |
| ELISA Type | Sandwich |
ATG5 / Autophagy protein 5, in combination with autophagy protein 12, functions as an E1-like activating enzyme in a ubiquitin-like conjugating system. ATG5 / Autophagy protein 5 is involved in several cellular processes, including autophagic vesicle formation, negative regulation of the innate antiviral immune response, lymphocyte development and proliferation, MHC II antigen presentation, adipocyte differentiation, and apoptosis. Diseases associated with ATG5 / Autophagy protein 5 include Spinocerebellar Ataxia and Stomatitis.
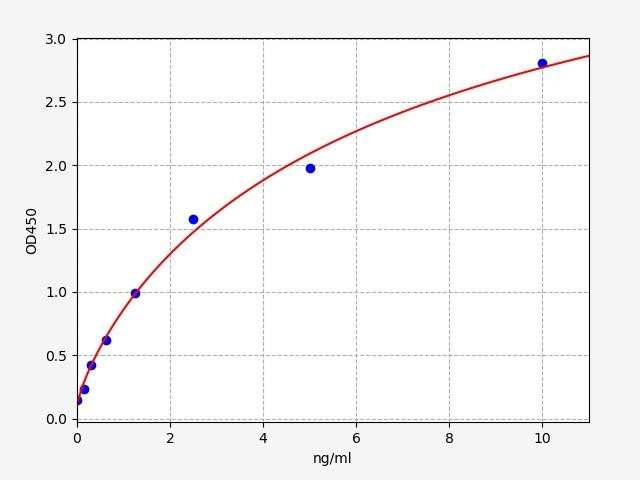
| Human ATG7(Autophagy-related protein 7) ELISA Kit | |
|---|---|
| Sensitivity | 0.094ng/ml |
| Range | 0.156-10ng/ml |
| ELISA Type | Sandwich |
ATG7 is a fundamental component of the autophagic machinery, playing a pivotal role in autophagy induction and execution by mediating the conjugation of LC3 and phosphatidylethanolamine.
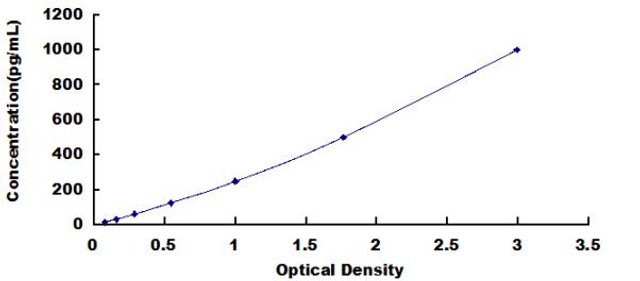
| Human Autophagy Related Protein 3 (ATG3) ELISA Kit | |
|---|---|
| Sensitivity | 6.9pg/mL |
| Range | 15.6-1000pg/mL |
| ELISA Type | Sandwich |
ATG3, also known as ubiquitin-like-conjugating enzyme ATG3, is essential for autophagosome formation, a critical step in the autophagy process that facilitates the degradation and recycling of cellular components.
Significance and Applications of Autophagy
The significance of autophagy extends far beyond its role in cellular maintenance. This intricate process plays a critical role in diverse physiological and pathological contexts, showcasing its relevance across various scientific disciplines.
Cellular Quality Control and Homeostasis: Autophagy acts as a meticulous quality control system, ensuring the removal of dysfunctional organelles, misfolded proteins, and cellular debris. This function is paramount for maintaining cellular homeostasis and preventing the accumulation of cellular waste that can contribute to aging and disease. By recycling these components, autophagy provides the cell with a constant supply of vital nutrients and energy precursors.
Metabolic Adaptation and Stress Response: In times of nutrient scarcity or stress, autophagy becomes a survival mechanism for cells. Through selective degradation and recycling, the cell can adapt to challenging conditions and sustain its essential functions. Autophagy's role in recycling cellular components can contribute to energy conservation, allowing cells to endure periods of adversity and optimize metabolic pathways.
Immune Regulation and Pathogen Defense: Autophagy is intimately linked to the immune response, as it can engulf and degrade intracellular pathogens such as viruses and bacteria. This not only helps in eliminating infections but also generates antigenic fragments that aid in activating immune responses against these invaders. Moreover, autophagy influences the presentation of self-antigens, playing a crucial role in immune tolerance and autoimmunity prevention.
Cancer and Neurodegenerative Diseases: Defects in autophagy have been implicated in the development of various diseases, including cancer and neurodegenerative disorders. Dysregulated autophagy can lead to the accumulation of toxic aggregates, contributing to conditions such as Alzheimer's, Parkinson's, and Huntington's diseases. Conversely, enhancing autophagy has shown promise as a therapeutic strategy in some cancer types by depriving tumor cells of resources and promoting their demise.
Therapeutic Potential and Future Directions: Given its multifaceted impact on health and disease, autophagy has garnered significant attention as a potential therapeutic target. Researchers are actively investigating ways to modulate autophagy for therapeutic purposes, either by enhancing its activity to combat diseases like cancer or by inhibiting it to alleviate neurodegenerative conditions. Clinical trials and experimental studies are exploring these strategies, and the results hold promise for novel treatments in the future.
As a result, autophagy emerges as a crucial process in cellular biology, directing a complex web of processes that regulate cellular quality control, metabolic adaptability, and immune surveillance. The cell's exceptional potential to adapt and survive in a variety of environments is demonstrated by its capacity to selectively breakdown and recycle cellular components. The significance of autophagy in health and disease is becoming increasingly clear, underscoring its function as a dynamic regulatory system that controls cell destiny and preserves organismal balance.
The complex interactions between autophagy and other physiological functions show the promise of autophagy as a therapeutic target in a range of medical specialties. The possibility of leveraging autophagy for disease intervention grows more real as researchers spend more time understanding the underlying molecular mechanisms and regulatory networks. Autophagy continues to capture the attention of the scientific community, providing a profound view into the complexity that underlay the tapestry of life. It has the potential to advance our understanding of fundamental cellular processes and pave the way for novel treatment approaches.
Written by Pragna Krishnapur
Pragna Krishnapur completed her bachelor degree in Biotechnology Engineering in Visvesvaraya Technological University before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026




