Fletikumab: Unlocking the Potential of Anti-CD47 Therapy in Cancer Research
Quick Facts About Fletikumab
What is Fletikumab?
How Does Fletikumab Work?
What Are the Clinical Applications of Fletikumab?
Is Fletikumab Safe?
1.) Understanding Fletikumab
Fletikumab represents a significant advancement in immunotherapy by targeting CD47, an immune checkpoint that plays a critical role in immune evasion by cancer cells. CD47 interacts with signal regulatory protein alpha (SIRPα) on macrophages, transmitting a "don’t eat me" signal that prevents immune cells from attacking malignant cells. Many cancers, including hematologic malignancies and solid tumors, exploit this pathway to escape immune surveillance. By blocking this interaction, Fletikumab restores macrophage activity and enhances the immune system’s ability to eliminate cancer cells.
The therapeutic potential of Fletikumab has been particularly notable in aggressive cancers such as acute myeloid leukemia (AML), non-Hodgkin’s lymphoma, and various solid tumors resistant to conventional treatments. Preclinical studies have demonstrated that CD47 inhibition leads to increased phagocytosis of cancer cells, improved antigen presentation, and enhanced T-cell responses, especially when used in combination with immune checkpoint inhibitors or chemotherapy.
Despite its promise, challenges remain in optimizing Fletikumab’s clinical applications. CD47 is expressed not only on cancer cells but also on normal red blood cells, leading to potential off-target effects such as anemia. Researchers are actively exploring strategies to minimize these adverse effects, including dosing adjustments, intermittent administration, and combination therapies that selectively enhance anti-tumor immunity while preserving normal hematologic function.
Ongoing clinical trials aim to refine patient selection criteria, ensuring that those most likely to benefit from CD47 blockade receive optimal treatment. By leveraging novel combinations with existing immunotherapies and targeted agents, Fletikumab represents a promising avenue for patients with refractory or relapsed cancers, addressing a critical unmet need in oncology.
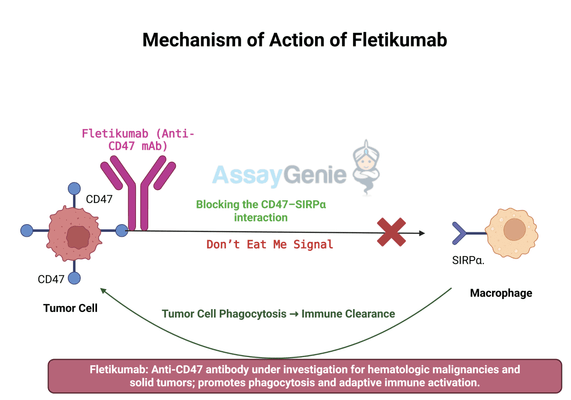
2.) Mechanism of Action of Fletikumab
Fletikumab works by disrupting the interaction between CD47, a transmembrane protein overexpressed on cancer cells, and SIRPα, a receptor found on macrophages and dendritic cells. Under normal physiological conditions, this interaction prevents excessive immune cell activation, ensuring that healthy cells are not mistakenly targeted for destruction. However, cancer cells hijack this mechanism by overexpressing CD47, effectively shielding themselves from immune attack.
By inhibiting CD47, Fletikumab removes this protective signal, allowing macrophages to recognize and engulf cancer cells through phagocytosis. This mechanism is particularly beneficial in hematologic malignancies, where immune evasion is a major contributor to disease progression. In addition to its direct impact on macrophages, Fletikumab has been shown to enhance dendritic cell activity, improving antigen presentation and stimulating adaptive immune responses, including cytotoxic T-cell activation.
One of the key advantages of Fletikumab’s mechanism is its potential for synergy with other immunotherapies. Preclinical models suggest that CD47 blockade enhances responses to PD-1/PD-L1 inhibitors by increasing immune cell infiltration into tumors. This has led to ongoing investigations into combination strategies involving Fletikumab and checkpoint inhibitors, chemotherapy, and antibody-drug conjugates to maximize therapeutic efficacy.
However, CD47 is also present on non-malignant cells, including erythrocytes and stem cells, posing a challenge for selective targeting. To mitigate hematologic toxicity, researchers are exploring various strategies such as engineering Fletikumab to preferentially bind tumor cells with minimal impact on red blood cells. Ongoing clinical trials aim to determine the optimal dosing regimen, balance efficacy with safety, and establish biomarkers that predict patient response to CD47 inhibition.
3.) Clinical Applications of Fletikumab
Fletikumab is being actively investigated for its therapeutic potential across various cancer types, particularly in hematologic malignancies and certain solid tumors. One of its primary applications is in the treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), diseases where immune evasion contributes significantly to disease progression. Studies have shown that CD47 blockade enhances the effectiveness of existing treatments, such as hypomethylating agents and chemotherapy, by making tumor cells more susceptible to immune-mediated destruction.
Beyond hematologic cancers, Fletikumab is also under investigation for solid tumors, including ovarian, colorectal, and lung cancers. In these malignancies, tumor cells often exhibit immune resistance, limiting the effectiveness of standard therapies. Early clinical trials have demonstrated that combining Fletikumab with checkpoint inhibitors like anti-PD-1 or anti-CTLA-4 therapies can overcome resistance mechanisms, increasing response rates and improving overall survival in patients with aggressive or refractory tumors.
Despite these promising findings, clinical development is ongoing, with researchers focusing on refining dosing strategies to minimize side effects. One of the main challenges is managing anemia, a known adverse effect of CD47 inhibition due to its expression on red blood cells. Strategies such as intermittent dosing, antibody modifications, and patient-specific treatment plans are being explored to optimize safety profiles while maintaining therapeutic efficacy.
Current clinical trials are also assessing biomarkers that could predict which patients are most likely to benefit from Fletikumab, enabling more personalized treatment approaches. As research progresses, Fletikumab holds the potential to become a cornerstone of cancer immunotherapy, particularly for patients who do not respond to conventional treatments or who develop resistance over time.

4.) Exploring Biosimilars for Fletikumab
What is a Biosimilar?

| Fletikumab (Anti-IL20) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-20 |
| Reactivity: | Human |
How Does a Fletikumab Biosimilar Compare?
Benefits of a Fletikumab Biosimilar in Research
- Cost-Effective Discovery: Facilitates drug research without the high costs associated with proprietary therapeutics.
- Preclinical Modeling: Supports the study of CD47 inhibition and immune responses in various cancer models.
- Combination Therapy Testing: Enables exploration of synergistic effects with other immunotherapeutic agents.
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Miren Ruiz de Eguilaz, PhD
Miren Ruiz de Eguilaz, PhD, has an extensive academic background, earning a BSc in Biology from UPV/EHU, an MSc in Biotechnology from the University of Oviedo, and a PhD in Chemistry from Dublin City University (DCU). Miren’s expertise lies in biosensor technology and bacterial diagnostics. She currently serves as a Product Manager at Assay Genie.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026