Lirilumab: Unlocking the Potential of Anti-KIR Therapy in Cancer Research
Quick Facts About Lirilumab
What is Lirilumab?
How Does Lirilumab Work?
What Are the Clinical Applications of Lirilumab?
1.) Understanding Lirilumab
Lirilumab, a monoclonal antibody developed by Bristol Myers Squibb in collaboration with Innate Pharma, is designed to target killer-cell immunoglobulin-like receptors (KIRs) on natural killer (NK) cells. NK cells play a crucial role in the innate immune system by recognizing and eliminating tumor cells or virally infected cells. However, many cancers exploit inhibitory KIR signaling to evade immune detection, allowing tumors to grow unchecked. By blocking these receptors, Lirilumab disrupts this immune evasion mechanism, effectively restoring NK cell functionality and enhancing their ability to target malignant cells.
Early preclinical studies and initial clinical trials demonstrated encouraging results, particularly in hematologic malignancies such as acute myeloid leukemia (AML) and multiple myeloma. The rationale for KIR blockade in cancer treatment was based on the hypothesis that removing inhibitory signals would improve NK cell cytotoxicity, leading to better tumor control. However, despite its promising mechanism, the clinical development of Lirilumab has encountered several challenges. In particular, combination strategies with immune checkpoint inhibitors, such as Nivolumab (anti-PD-1), did not yield the expected synergistic benefits in some studies. Researchers are now focusing on refining patient selection criteria and exploring alternative therapeutic approaches to maximize the effectiveness of KIR inhibition.
While Lirilumab has not yet secured regulatory approval, its role in immuno-oncology continues to be an area of active investigation. Understanding the complexities of NK cell regulation and the interplay between inhibitory and activating receptors is crucial for optimizing next-generation KIR-targeting therapies.
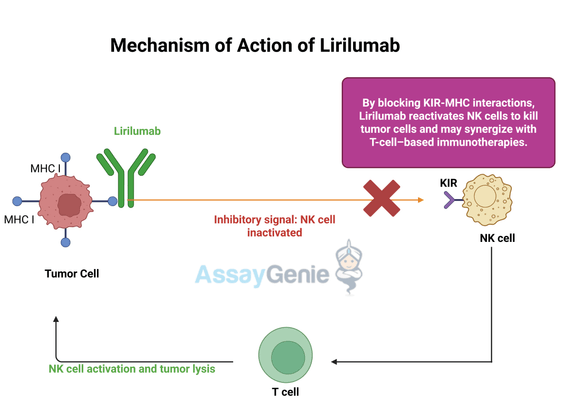
2.) Mechanism of Action of Lirilumab
Lirilumab functions by selectively inhibiting KIR receptors, a key regulatory component of NK cell activity. KIR receptors interact with major histocompatibility complex (MHC) class I molecules on healthy cells, transmitting inhibitory signals that prevent NK cells from attacking normal tissues. This mechanism is essential for immune self-tolerance. However, many tumor cells retain MHC class I expression, enabling them to escape NK cell-mediated destruction by exploiting KIR signaling to suppress immune responses.
By blocking KIR-MHC interactions, Lirilumab lifts this suppression, effectively reactivating NK cells and enhancing their cytotoxic potential. This makes Lirilumab a promising candidate for cancer immunotherapy, particularly in malignancies where NK cell dysfunction contributes to disease progression. Preclinical studies have shown that Lirilumab can enhance tumor cell lysis by increasing NK cell activation and cytokine production, thereby boosting anti-tumor immunity.
One of the most compelling aspects of Lirilumab's mechanism is its potential synergy with other immunotherapies, especially immune checkpoint inhibitors like Nivolumab (anti-PD-1) and Ipilimumab (anti-CTLA-4). The rationale behind these combinations is that while checkpoint inhibitors primarily enhance T-cell-mediated responses, Lirilumab simultaneously boosts NK cell activity, offering a dual-pronged approach to tumor elimination. However, clinical trials investigating these combinations have yielded mixed results, suggesting that additional factors—such as tumor microenvironment dynamics, patient immune profiles, and dosing strategies—may influence therapeutic outcomes.
Despite these challenges, the concept of targeting inhibitory KIRs remains a significant avenue in cancer immunotherapy research. Ongoing studies are focused on optimizing Lirilumab’s use in specific patient populations and identifying biomarkers that predict responsiveness to KIR blockade.
3.) Clinical Applications of Lirilumab
Lirilumab has been investigated in several clinical trials, primarily for hematologic malignancies and solid tumors, including acute myeloid leukemia (AML), multiple myeloma, and non-small cell lung cancer (NSCLC). Given its mechanism of action, Lirilumab was hypothesized to enhance NK cell-mediated tumor clearance, particularly in cancers where immune evasion through inhibitory KIR signaling plays a significant role.
In AML, early-phase studies suggested that Lirilumab could improve outcomes when combined with other immunotherapeutic agents or standard chemotherapy. However, later trials indicated that while KIR blockade was well-tolerated, its efficacy as a monotherapy or in combination regimens did not meet expectations. As a result, some clinical programs evaluating Lirilumab in AML were discontinued, highlighting the need for better patient stratification and combination strategies.
Similarly, Lirilumab was explored in multiple myeloma, where NK cells play a role in controlling disease progression. However, clinical trials faced challenges in demonstrating a significant benefit over existing therapies, leading researchers to reevaluate its optimal use. In NSCLC, Lirilumab was tested in combination with Nivolumab, based on the hypothesis that dual immune modulation could enhance anti-tumor responses. While this approach initially showed promise, large-scale studies did not confirm a clear clinical advantage, underscoring the complexities of immunotherapy combinations.
Despite these setbacks, ongoing research continues to explore ways to optimize KIR blockade, including patient selection based on immune profiling, alternative dosing strategies, and next-generation NK cell-targeting approaches. Lirilumab's development has provided valuable insights into NK cell biology, paving the way for future immunotherapies targeting innate immune pathways.

4.) Exploring Biosimilars for Lirilumab
What is a Biosimilar?
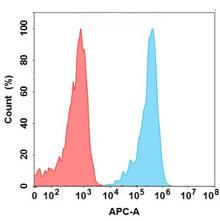
| Lirilumab (Anti-KIR2DL2) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | KIR2DL2 |
| Reactivity: | Human |
How Does a Lirilumab Biosimilar Compare to the Original?
Benefits of Lirilumab Biosimilars in Research
- Cost-Effective Research Tool: Provides researchers with a viable alternative for studying anti-KIR mechanisms.
- Consistent Performance: Ensures reproducibility in preclinical and translational research.
- Supports Drug Development: Enables further exploration of KIR blockade strategies without reliance on proprietary compounds.
Research Use Only Disclaimer:
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
The Rise of Alpha-Emitting Radiopharmaceuticals: A New Era in Targeted Cancer Therapy
Imagine a microscopic sniper, capable of delivering a lethal blow to a single cancer cell while lea …19th Feb 2026 -
Targeted Protein Degradation: The Next Frontier in Oncology Drug Discovery
For decades, cancer drug development has focused on blocking proteins—inhibiting enzymes, antagoniz …18th Feb 2026 -
Neuroinflammation and Compulsive Behavior: The Role of Astrocytes in OCD
Imagine a mind trapped in a loop, endlessly repeating actions or thoughts, driven by an invisible f …18th Feb 2026