Milatuzumab: Unveiling Its Role in Cancer Immunotherapy and Research
What You Need to Know About Milatuzumab
What is Milatuzumab?
Milatuzumab is a humanized monoclonal antibody targeting CD74, a molecule involved in antigen presentation and cell signaling.
What is the mechanism of action for Milatuzumab?
Milatuzumab binds CD74, interrupting survival pathways and triggering antibody-dependent cell-mediated cytotoxicity (ADCC).
Is Milatuzumab safe?
Milatuzumab has shown manageable safety in early trials, though further studies are needed to fully define its toxicity profile.
What diseases is Milatuzumab being studied for?
Milatuzumab has been investigated in hematological malignancies like multiple myeloma and chronic lymphocytic leukemia, and autoimmune disorders such as systemic lupus erythematosus.
1.) Understanding Milatuzumab
Milatuzumab is a pioneering humanized monoclonal antibody specifically designed to target CD74, a transmembrane glycoprotein primarily expressed on antigen-presenting immune cells such as B cells, monocytes, and macrophages. CD74 plays a dual role—acting as an MHC class II chaperone facilitating antigen presentation and as a signaling molecule involved in cell survival, proliferation, and inflammation. In various malignancies, including multiple myeloma, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL), CD74 is markedly overexpressed, making it a compelling therapeutic target.
Developed originally by Immunomedics, Milatuzumab demonstrated promising preclinical and early clinical activity by directly inducing apoptosis and enhancing cytotoxicity in CD74-expressing cells. Its specificity allows it to penetrate the tumor microenvironment effectively, targeting malignant cells while sparing healthy tissues. Moreover, CD74’s involvement in immune regulation has led researchers to investigate Milatuzumab’s utility beyond oncology, particularly in autoimmune diseases such as systemic lupus erythematosus (SLE), where abnormal B-cell activity plays a key pathogenic role.
Although some clinical development programs for Milatuzumab have been discontinued, the antibody remains a critical proof-of-concept molecule. It has served as a model for developing next-generation CD74-targeted therapies and antibody-drug conjugates (ADCs). Its capacity to influence both tumor biology and immune dynamics positions it well for integration into combination regimens with chemotherapy, immune checkpoint inhibitors, or other targeted agents. The insights gained from Milatuzumab continue to inform therapeutic strategies aimed at modulating the immune system in both cancer and autoimmune disorders, underlining its scientific and clinical significance.
2.) Milatuzumab Mechanism of Action
Milatuzumab exerts its therapeutic effects by specifically binding to CD74, a cell surface molecule with key roles in immune regulation and oncogenic signaling. CD74 serves not only as a chaperone for MHC class II molecules but also as a high-affinity receptor for macrophage migration inhibitory factor (MIF), a cytokine involved in promoting tumor growth, immune evasion, and inflammation. The CD74-MIF interaction activates downstream signaling cascades that enhance cell survival and proliferation, particularly in hematologic malignancies such as multiple myeloma, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL), where CD74 is highly expressed.
Milatuzumab disrupts this pathological axis through multiple mechanisms. First, it blocks the MIF-CD74 interaction, thereby inhibiting key pro-survival pathways such as the PI3K/Akt and NF-κB signaling cascades. This interference diminishes the tumor cells’ ability to resist apoptosis. Second, Milatuzumab directly induces apoptosis by interfering with cellular homeostasis in malignant cells, contributing to tumor regression. Third, it activates immune effector functions by promoting antibody-dependent cellular cytotoxicity (ADCC) and enhancing macrophage-mediated phagocytosis, leading to more efficient clearance of cancer cells.
These combined effects underscore Milatuzumab’s potential as a multifaceted therapeutic agent. Importantly, its ability to reshape the tumor immune microenvironment—by reducing immune suppression and enhancing anti-tumor immune activity—makes it an attractive candidate for combination therapies. In particular, pairing Milatuzumab with immune checkpoint inhibitors could amplify the therapeutic response, especially in immunologically “cold” tumors. Overall, Milatuzumab's mechanistic versatility offers a compelling platform for targeted immuno-oncology strategies.
3.) Clinical Applications of Milatuzumab
Milatuzumab has been extensively investigated in hematologic malignancies, particularly multiple myeloma, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL), where CD74 is significantly overexpressed. In multiple myeloma, the expression of CD74 on malignant plasma cells provided a compelling rationale for therapeutic targeting. Preclinical models and early-phase clinical trials indicated that Milatuzumab could directly inhibit tumor cell proliferation and sensitize malignant cells to chemotherapeutic agents, thereby enhancing overall treatment efficacy.
In CLL and NHL, Milatuzumab demonstrated further potential, especially when used in combination with standard chemotherapeutics such as doxorubicin. These combinations appeared to produce additive or synergistic effects, leading to improved anti-tumor responses. Additionally, Milatuzumab was explored as part of antibody-drug conjugate (ADC) strategies, where its specificity for CD74 enabled the targeted delivery of cytotoxic agents directly to cancer cells, minimizing off-target effects and maximizing therapeutic precision.
Beyond oncology, Milatuzumab’s role as an immune modulator garnered interest in autoimmune disease contexts, particularly systemic lupus erythematosus (SLE). In this setting, its ability to deplete or regulate B-cell populations—central players in autoantibody production—highlighted its broader applicability in immune-mediated disorders.
Despite the discontinuation or pause of some clinical development programs, Milatuzumab remains an important asset in immunological and oncological research. The wealth of data generated from its development has provided a foundational understanding of CD74 as a therapeutic target. This knowledge continues to guide the design of newer biologics and combination therapies that aim to harness or improve upon Milatuzumab’s mechanisms of action in both cancer and autoimmune diseases.
4.) Advancing Research on Magrolimab with Biosimilars
What is a Biosimilar?
A biosimilar is a biologic product that closely mimics the reference (original) biologic in terms of safety, purity, and potency. Biosimilars are crucial tools for researchers, offering accessible alternatives for preclinical and discovery applications.
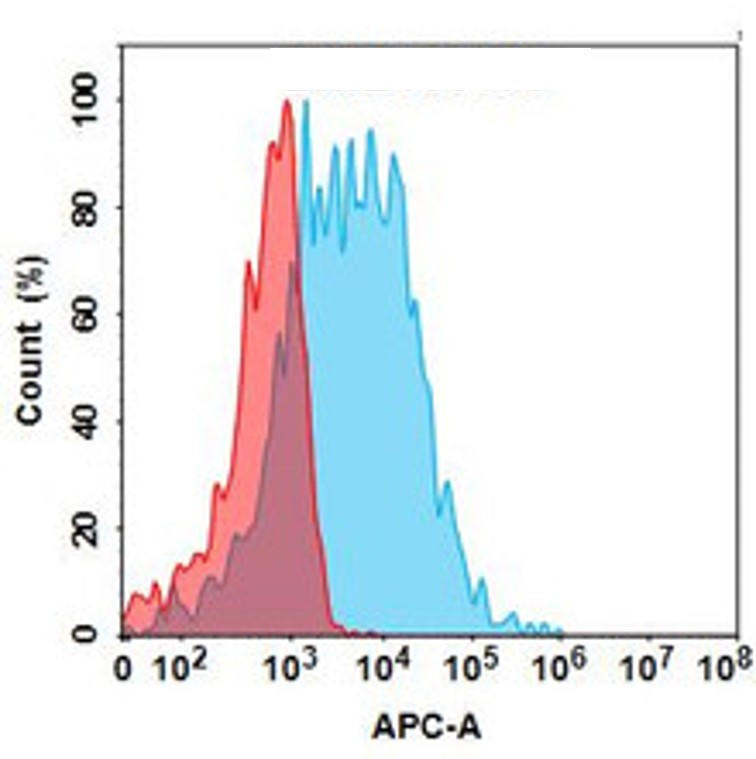
| Milatuzumab (Anti-CD74) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CD74 |
| Reactivity: | Human |
How Does the Magrolimab Biosimilar Compare?
Our biosimilar product, Milatuzumab biosimilar , replicates the binding specificity and biological behavior of the original antibody. Designed for research use only, it provides consistent, reproducible results in lab environments.
Research Advantages of the Milatuzumab Biosimilar
· Reproducibility: Maintains high fidelity to the original antibody’s binding and functional characteristics.
· Cost-effective: Enables broader research access without reliance on clinical-grade material.
· Versatility: Useful in target validation, flow cytometry, and cell-based assays.
Research Use Only Disclaimer:
Our Milatuzumab Biosimilar Antibody is intended exclusively for research use and is not approved for clinical or therapeutic applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026



