Optimizing Organoid Culture Conditions: Paving the Way for Revolutionary Advances in Biomedical Research
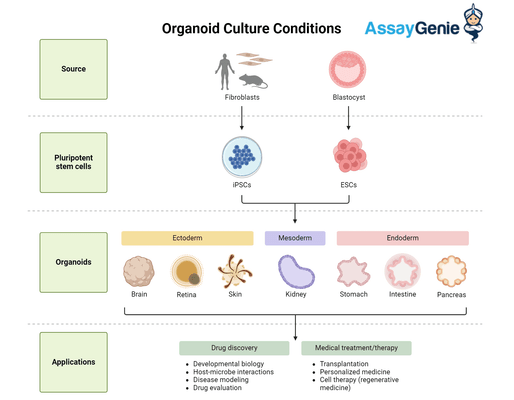
In the dynamic landscape of biomedical research, organoids have emerged as a groundbreaking tool, offering three-dimensional (3D) models that mimic the complex architecture and functionality of human organs. These miniature, self-organizing structures have revolutionized our approach to understanding human development, disease modeling, and drug discovery. However, the key to harnessing their full potential lies in optimizing organoid culture conditions. This intricate process involves fine-tuning the biochemical and physical environment to support the growth, differentiation, and maturation of organoids. This article delves into the critical aspects of organoid culture, including the selection of stem cells, the role of the extracellular matrix (ECM), the importance of growth factors and signaling molecules, and the challenges and future directions in the field.
Stem Cell Selection: The Foundation of Organoid Culture
The journey of organoid culture begins with the selection of appropriate stem cells. Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), offer a versatile starting point due to their ability to differentiate into any cell type. Alternatively, organ-specific adult stem cells can be used to generate organoids that closely resemble the tissue of origin. The choice between PSCs and adult stem cells depends on the research objective, with PSCs being preferred for developmental studies and adult stem cells for modeling mature organ function.
The Extracellular Matrix: A Scaffold for Growth
The ECM plays a pivotal role in organoid culture, providing a scaffold that supports cell attachment, proliferation, and spatial organization. Hydrogels derived from natural ECM components, such as Matrigel and collagen, are commonly used to emulate the organ-specific microenvironment. These matrices not only support the structural integrity of organoids but also modulate cell behavior through biochemical cues and mechanical properties. Recent advances focus on developing synthetic, tunable ECMs that offer greater reproducibility and the possibility to systematically study the effects of ECM composition on organoid development.
Growth Factors and Signaling Molecules: Steering Organoid Development
The addition of growth factors and signaling molecules to the culture medium is essential for directing the differentiation and maturation of stem cells into specific organ cell types. These molecules mimic the signaling pathways active during organ development, guiding the self-organization of cells into functional organoids. The composition and concentration of these factors must be meticulously adjusted to replicate the organ-specific niche, a process that often involves empirical optimization to achieve the desired organoid phenotype.
Oxygen and Nutrient Supply: Meeting Metabolic Demands
Ensuring adequate oxygen and nutrient supply is critical for the viability and growth of organoids. Traditional static culture systems can lead to gradients of oxygen and nutrients, with cells at the organoid periphery experiencing different conditions than those at the core. This issue has been addressed through the development of dynamic culture systems, such as spinning bioreactors and microfluidic devices, which improve mass transfer and mimic physiological flow, thereby promoting more uniform organoid growth and differentiation.
Challenges in Organoid Culture:
Despite significant progress, several challenges remain in organoid culture. These include variability in organoid size and morphology, the complexity of replicating the complete cellular diversity of organs, and the integration of vascular-like networks to support larger, more complex organoids. Addressing these challenges requires innovative approaches in biomaterials science, stem cell biology, and bioengineering.
Future Directions: Towards Personalized Medicine and Beyond
Looking ahead, the optimization of organoid culture conditions holds promise for personalized medicine, enabling the generation of patient-specific organoids for disease modeling, drug screening, and regenerative therapies. Advances in genome editing technologies, such as CRISPR-Cas9, further expand the potential of organoids to model genetic diseases and screen for therapeutic agents. Additionally, the integration of organoids with other technologies, such as organ-on-a-chip systems and machine learning algorithms, opens new avenues for high-throughput drug testing and the development of more accurate models of human physiology and disease.
Conclusion
References
- Lancaster, M.A., & Knoblich, J.A. (2014). Generation of cerebral organoids from human pluripotent stem cells. Nature Protocols, 9(10), 2329-2340.
- Clevers, H. (2016). Modeling Development and Disease with Organoids. Cell, 165(7), 1586-1597.
- Takebe, T., Sekine, K., Enomura, M., Koike, H., Kimura, M., Ogaeri, T., Zhang, R.R., Ueno, Y., Zheng, Y.W., Koike, N., Aoyama, S., Adachi, Y., & Taniguchi, H. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature, 499(7459), 481-484.
- Drost, J., & Clevers, H. (2018). Organoids in cancer research. Nature Reviews Cancer, 18(7), 407-418.
- Gjorevski, N., Sachs, N., Manfrin, A., Giger, S., Bragina, M.E., Ordóñez-Morán, P., Clevers, H., & Lutolf, M.P. (2016). Designer matrices for intestinal stem cell and organoid culture. Nature, 539(7630), 560-564.
- Huch, M., Gehart, H., van Boxtel, R., Hamer, K., Blokzijl, F., Verstegen, M.M.A., Ellis, E., van Wenum, M., Fuchs, S.A., de Ligt, J., van de Wetering, M., Sasaki, N., Boers, S.J., Kemperman, H., de Jonge, J., Ijzermans, J.N.M., Nieuwenhuis, E.E.S., Hoekstra, R., Strom, S., Vries, R.G., van der Laan, L.J.W., & Clevers, H. (2015). Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell, 160(1-2), 299-312.
- Kim, J., Koo, B.K., & Knoblich, J.A. (2020). Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biology, 21(10), 571-584.
- Rossi, G., Manfrin, A., & Lutolf, M.P. (2018). Progress and potential in organoid research. Nature Reviews Genetics, 19(11), 671-687.
Written by Tehreem Ali
Tehreem Ali completed her MS in Bioinformatics and conducted her research work at the IOMM lab at GCUF, Pakistan.
Recent Posts
-
What Are Oligodendrocytes? Functions, Markers & Disease Links
What Are Oligodendrocytes? Functions, Markers & Disease LinksOligodendrocytes are pivo …24th Sep 2025 -
Complete T Helper Cell Guide: Th1, Th2, Th17 & Functions - Your Ultimate Resource from Assay Genie
Complete T Helper Cell Guide: Th1, Th2, Th17 & Functions - Your Ultimate Resource from …27th Aug 2025 -
Apoptosis Unveiled: Your Complete Guide to Intrinsic & Extrinsic Pathways
Apoptosis Unveiled: Your Complete Guide to Intrinsic & Extrinsic PathwaysAt Assay Geni …27th Aug 2025