The p53 Protein: Tumor Suppressor Protein
p53, encoded by the TP53 gene on chromosome 17, is a fundamental protein safeguarding against tumor formation. It orchestrates cellular responses to DNA damage and stress, crucially managing cell division. By activating a range of mechanisms like cell cycle regulation, DNA repair, apoptosis, and senescence, p53 maintains cellular integrity and prevents cancerous growths.
Key Takeaways:
- p53, encoded by the TP53 gene, is a critical protein for cell division regulation and cancer prevention.
- It responds to DNA damage and stress by controlling cell cycle, DNA repair, and apoptosis.
- p53's structure includes various domains, each with specific functions.
- Dysregulated p53 activity, often due to mutations, is linked to cancer development.
- Monitoring p53 levels is essential in cancer research for understanding and targeting its role in tumors.
What is p53?
p53 is a crucial protein involved in the regulation of cell division and serves as a key protector against the formation of cancerous tumors. It is encoded by the TP53 gene, which is located on chromosome 17. Upon encountering cellular stress signals or DNA damage, p53 is activated, initiating a cascade of cellular responses. These responses encompass a wide range of intricate functions, including the precise regulation of the cell cycle, facilitation of DNA repair mechanisms, induction of programmed cell death, and promotion of cellular growth arrest (senescence).
p53 Protein Structure
The p53 protein is composed of four chains of 393 amino acids each, forming an active tetramer. It consists of several distinct domains with specific functions.
-
N-Terminal Transactivation Domain I (TADI) and N-Terminal Transactivation Domain II (TADII):
Located at the N-terminus of p53, the protein possesses two transactivation domains: TADI and TADII. These domains are responsible for activating gene transcription. By interacting with various transcriptional co-activators and the general transcription machinery, TADI and TADII initiate the expression of target genes involved in vital cellular processes such as cell cycle control, DNA repair, and apoptosis. -
Proline-rich Domain (PRD): The proline-rich domain is involved in protein-protein interactions. It enables p53 to interact with other transcription factors, coactivators, and corepressors, thereby modulating its transcriptional activity.
-
Central DNA-binding Domain (DBD): The central DNA-binding domain is the most well-known and highly conserved region of p53. It consists of a sequence-specific DNA-binding domain (DBD) responsible for recognizing and binding to specific DNA sequences known as p53 response elements (p53REs). The binding of p53 to p53REs regulates the transcription of target genes.
-
Tetramerization Domain (TD): The tetramerization domain allows p53 to form a stable tetramer, which is the active form of the protein. It is crucial for the proper folding and stability of p53 and is involved in mediating protein-protein interactions with other p53 molecules.
-
C-terminal Regulatory Domain (CTD): The C-terminal regulatory domain contains multiple functional subdomains that play a role in regulating the stability, subcellular localization, and protein-protein interactions of p53. This region includes the nuclear export signal (NES), nuclear localization signal (NLS), and sites for post-translational modifications such as phosphorylation and acetylation
- Oligomerization Domain (OD): The oligomerization domain is involved in the formation of higher-order oligomeric complexes of p53. It plays a critical role in the stability and DNA-binding activity of p53 by facilitating the interaction between p53 monomers.
These domains work together to confer various functions to the p53 protein, allowing it to act as a central regulator of cellular responses to stress, DNA damage, and oncogenic signals.
p53 Signalling Pathway
The p53 signaling pathway is activated in response to cellular stress or DNA damage. When activated, p53 acts as a transcription factor, regulating the expression of various target genes. These target genes are involved in different cellular processes, such as cell cycle arrest, DNA repair, apoptosis, and senescence. By coordinating these processes, p53 helps maintain genomic stability and prevents the formation of cancerous tumors. The specific target genes regulated by p53 can vary depending on the context and the type of cellular stress. However, some key target genes include those involved in cell cycle control (e.g., CDKN1A/p21), DNA repair (e.g., GADD45, PUMA), apoptosis (e.g., BAX, PUMA), and senescence-associated factors (e.g., p16INK4a).
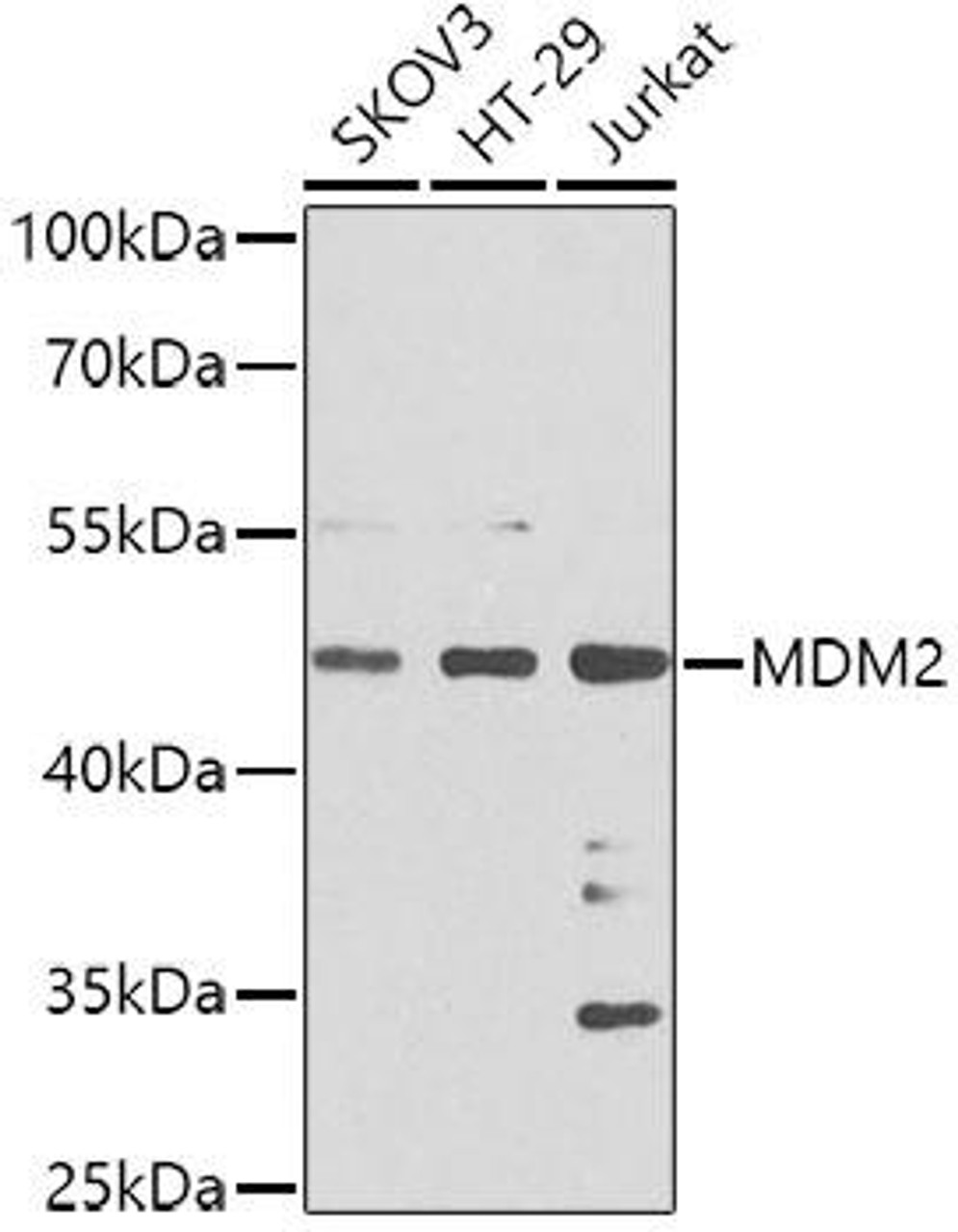
The p53 signaling pathway is activated by stress signals, and they can cause the release of p53 from its inhibitor, MDM2. Once released, p53 binds to DNA and promotes the transcription of genes that lead to cell cycle arrest or apoptosis. Cell cycle arrest allows damaged cells to repair their DNA before replicating, while apoptosis is a process of programmed cell death that prevents damaged cells from replicating and spreading mutations.
p53 is a key regulator of senescence, a process by which cells stop dividing and enter a state of permanent growth arrest. Senescent cells are thought to play an important role in the aging process and the development of age-related diseases.
p53 is also involved in the regulation of cell proliferation. In response to stress signals, p53 can induce cell cycle arrest or apoptosis. However, under normal conditions, p53 actually promotes cell proliferation by inhibiting senescence. This ensures that cells can continue to divide and proliferate in response to growth signals.
P53 Signalling
Regulation of p53
The p53 protein is tightly regulated to maintain proper cellular function. It is negatively regulated by MDM2 (mouse double minute 2), which inhibits p53's transcriptional activity and promotes its degradation. However, in response to cellular stress or DNA damage, signals disrupt the p53-MDM2 interaction, leading to p53 stabilization and activation. Various post-translational modifications, such as phosphorylation, acetylation, and methylation, further modulate p53 activity by affecting its stability, DNA-binding affinity, interaction with co-regulators, and target gene specificity. Other proteins and pathways, including ATM (ataxia telangiectasia mutated), ATR (ataxia telangiectasia and Rad3-related), and p14ARF (alternative reading frame), also contribute to p53 regulation by sensing cellular stress signals and either activating or stabilizing p53.
P53 Regulation by MDM2
p53 Target Genes
The specific genes regulated by p53 can vary depending on the context and the type of cellular stress. However, there are several key target genes that are commonly regulated by p53. These include:
-
CDKN1A/p21: This gene is involved in cell cycle control and acts as a regulator of cell proliferation. p53 activation can lead to the upregulation of CDKN1A/p21, resulting in cell cycle arrest.
-
GADD45: This gene plays a role in DNA repair and is activated by p53 in response to DNA damage. GADD45 aids in the repair of DNA lesions and maintains genomic stability.
-
BAX: p53 can induce the expression of BAX, a gene involved in apoptosis. BAX promotes programmed cell death and eliminates cells with severe DNA damage.
-
PUMA: Another gene involved in apoptosis, PUMA (p53 upregulated modulator of apoptosis) is regulated by p53 and promotes cell death in response to DNA damage.
-
p16INK4a: This gene is associated with senescence, a state of permanent growth arrest. p53 can regulate the expression of p16INK4a, contributing to cellular senescence.
The p53 protein regulates the expression of these genes in response to stress signals. Under normal conditions, p53 levels are low and these genes are expressed at low levels. However, when DNA is damaged or other stress signals are present, p53 levels increase and this leads to the increased expression of these genes. The proteins encoded by these genes then mediate the cell's response to the stress. This can lead to cell cycle arrest, DNA repair, or apoptosis.
How is p53 measured?
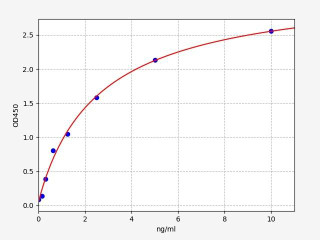
Quantifying p53 protein levels is achieved using various techniques in cancer research and diagnostics. Cell-based ELISA, western blotting, immunofluorescence, and immunohistochemistry are commonly employed to measure total p53 protein levels in cells or detect p53 protein in specific cell types or those undergoing apoptosis. ELISA, an immunoassay, enables the quantification of p53 protein levels, including both total and phosphorylated forms. These established methods provide insights into p53 expression, post-translational modifications, and interactions with other molecules. Specific kits and commercial assays are also widely used for standardized and reliable p53 measurement.
Example of a p53 standard curve
p53 Related Kits
| Human PUMA / BBC3 / P53 Upregulated Modulator of Apoptosis ELISA Kit | |
|---|---|
| ELISA Type | Sandwich |
| Sensitivity | 0.094ng/ml |
| Range | 0.156-10ng/ml |

| Human MTBP / Mdm2-Binding Protein ELISA Kit | |
|---|---|
| ELISA Type | Sandwich |
| Sensitivity | 0.188ng/ml |
| Range | 0.313-20ng/ml |

| Human MDMX ELISA Kit (HUFI07657) | |
|---|---|
| ELISA Type | Sandwich |
| Sensitivity | 0.094ng/ml |
| Range | 0.156-10ng/ml |
p53 and Cancer
Mutations in the TP53 gene, which encodes for p53, are commonly observed in various types of cancer. These mutations result in the loss or alteration of p53's tumor suppressor function, leading to the disruption of critical cellular processes and promoting tumorigenesis. Mutant p53 proteins often exhibit a gain of function, acquiring new oncogenic properties that contribute to tumor growth, invasion, and metastasis. Moreover, p53 mutations are associated with poor prognosis and resistance to conventional cancer therapies. Understanding the impact of p53 mutations in different cancer types is crucial for developing targeted therapies that specifically address the aberrant p53 signaling and restore normal cellular functions to combat cancer effectively.
p53 is a critical tumor suppressor protein that plays a vital role in preventing cancer. It safeguards the genome by initiating responses to DNA damage. Upon activation, p53 promotes cell cycle arrest to allow for DNA repair or induces apoptosis if the damage is irreparable. Mutations in the p53 gene are commonly found in various cancers, leading to dysfunctional p53. Mutant p53 proteins can interfere with the function of wild-type p53 or other tumor suppressors, contributing to tumor development and progression.
Dysfunctional p53 disrupts key cellular processes involved in tumor suppression. It influences angiogenesis, the formation of blood vessels that supply tumors, and cellular metabolism, impacting the balance between glycolysis and oxidative phosphorylation. Consequently, p53 mutations are associated with poor prognosis, therapy resistance, and increased tumor recurrence rates in cancer patients. However, targeting mutant p53 holds promise for therapeutic interventions.
Written by Lauryn McLoughlin
Lauryn McLoughlin completed her undergraduate degree in Neuroscience before completing her masters in Biotechnology at University College Dublin.
Recent Posts
-
IgG1 Plasma Cells: The Emerging Biomarker for Predicting Cancer Immunotherapy Success
In the relentless fight against cancer, immunotherapy has emerged as a beacon of hope, harnessing t …24th Feb 2026 -
The Rise of Cancer Neuroscience: How Neural Circuits Drive Tumor Progression
For decades, we viewed cancer as a rogue army of cells, a biological glitch driven solely by geneti …23rd Feb 2026 -
CRISPR-Powered Light Sensors: A New Frontier in Ultra-Sensitive Cancer Detection
Cancer detection often relies on advanced imaging or invasive procedures, frequently catching the d …20th Feb 2026